Figure 1.
Cotranslational Folding of the ADR1a Zinc-Finger Domain
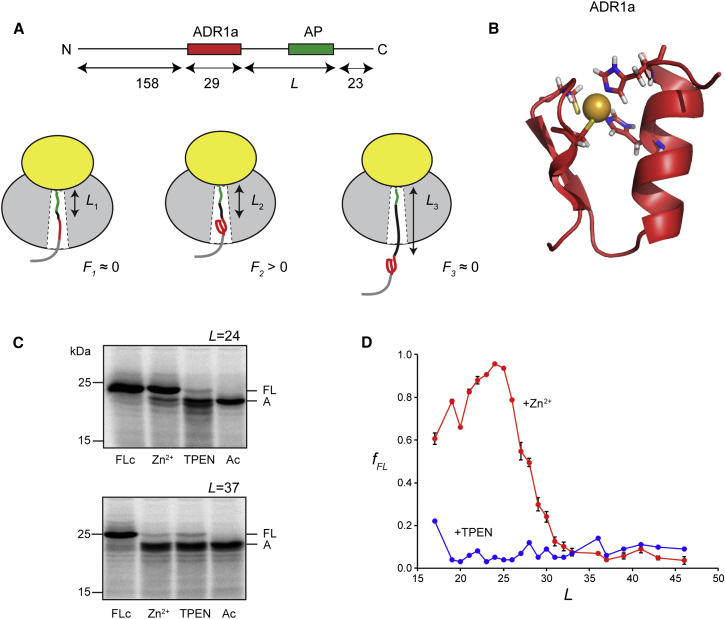
(A) Force measurement assay. The ADR1a domain is placed L residues away from the C-terminal Pro residue in the E. coli SecM AP. An unrelated segment from the E. coli LepB protein (LepB residues 78–226) is added to the N terminus in order to increase the size of the protein such that it can be readily visualized by SDS-PAGE, and a 23-residue C-terminal segment ensures that arrested and full-length forms of the protein can be easily separated on the gel. The LepB part is composed of five small β-hairpin segments that do not interact with one another in the LepB structure (PDB: 1B12) and hence cannot fold in itself. The cartoon below shows three ADR1a-AP constructs with different values of L (L1 < L2 < L3). The ribosomal tunnel is too tight for the protein to fold at L1, and the protein is already folded and outside the tunnel when the ribosome reasches the AP at L3. Only at L2 will folding of the protein against the widening ribosomal exit tunnel generate a pulling force F on the AP, leading to inefficient ribosomal stalling and an increase in the fraction full-length protein, fFL.
(B) Structure of ADR1a (PDB: 2ADR). The Zn2+ ion is shown in gold.
(C) In vitro translation the PURE system of the ADR1a-SecM (L = 24) (top) and ADR1a-SecM (L = 37) (bottom) constructs. Full-length (FL) and arrested (A) forms are indicated. Ac, control construct with a stop codon inserted directly after the AP; FLc, full-length control construct, where the critical Pro at the end of the AP is mutated to Ala; TPEN, translation carried out in the presence of 50 μM of the Zn2+ chelator TPEN; Zn2+, translation carried out in the presence of 50 μM Zn2+.
(D) Fraction full-length protein, fFL, plotted as a function of L for the ADR1a-AP constructs translated in the PURE in vitro system either in the absence (blue curve; to deplete the translation mix of Zn2+, the Zn2+ chelator TPEN was included at 50 μM) or presence (red curve) of 50 μM Zn2+. SEMs are indicated.
See also Figures S1 and S2.