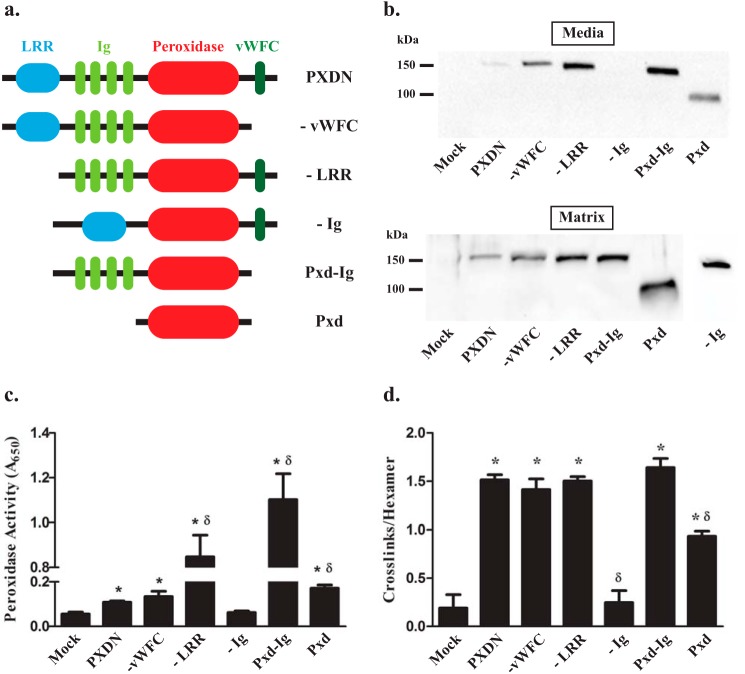
FIGURE 4.
The immunoglobulin and peroxidase domains of peroxidasin are required for sulfilimine bond formation. a, schematic depiction of the domain structure of peroxidasin and its deletion constructs. b, immunoblotting of media and matrix fractions under reducing conditions after transient transfection of overlaid HEK293T with peroxidasin constructs demonstrates adequate secretion and matrix incorporation of all constructs except the −Ig construct. Blots are representative of 5 independent experiments. c, all of the constructs, except −Ig, exhibit tetramethylbenzidine colorimetric peroxidase activity greater than mock transfection (*, p < 0.05; n = 7) with some constructs demonstrating peroxidase activity greater than wild type peroxidasin (δ, p < 0.05; n = 7). d, all of the constructs formed sulfilimine cross-links in collagen IV, quantified as cross-links per hexamer, better than mock control except for the −Ig construct (*, p < 0.05, n = 5). The catalytic domain only construct (Pxd) formed more cross-links than mock, but was significantly less than wild type Pxdn (δ, p < 0.05, n = 5). Data were analyzed using analysis of variance followed by post hoc pairwise comparisons with Tukey's correction for multiple comparisons.