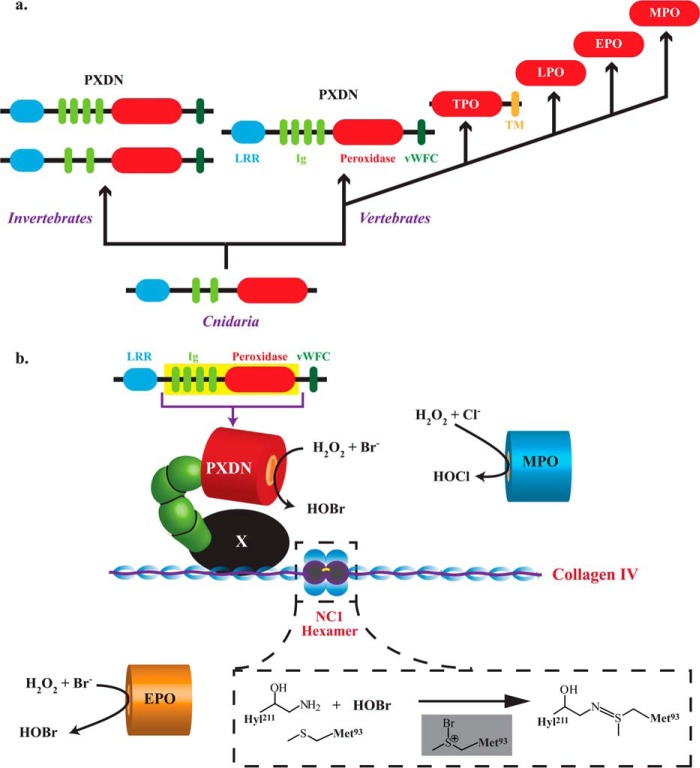
FIGURE 6.
Evolution of animal peroxidases and model for peroxidasin-mediated sulfilimine bond formation in collagen IV. a, PXDN is the ancestral animal heme peroxidase found in Cnidaria that extends into invertebrates and vertebrates with additional Ig and vWFC domains. In vertebrates, peroxidasin most likely gave rise to thyroid peroxidase (TPO) via gene duplication, deletion of non-catalytic domains, and fusion with a transmembrane (TM) domain. Lactoperoxidase (LPO), EPO, and MPO arose from successive gene duplications after loss of the TPO TM domain. b, the N-terminal Ig domain of peroxidasin is proposed to interact with an unknown intermediary matrix protein (in black denoted as X) to form a ternary complex with collagen IV such that HOBr generated by its catalytic domain is placed in a spatially productive position to form sulfilimine cross-links in the NC1 hexamer. Peroxidasin-derived HOBr oxidizes Met-93 to create a bromosulfonium intermediate leading to sulfilimine bond formation (inset). MPO and EPO associate with basement membrane but do not produce HOBr (MPO) or do so in a spatially inefficient manner (EPO).