FIGURE 6.
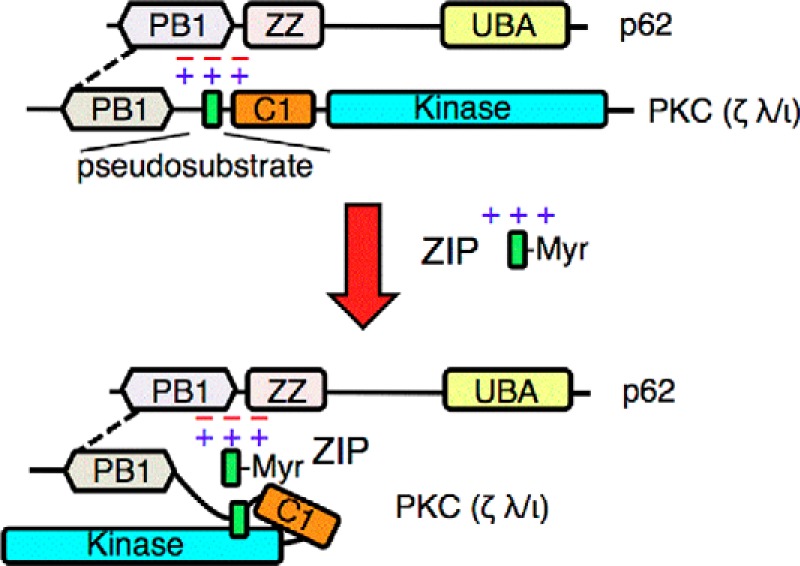
The acidic surface of p62 tethers the pseudosubstrate segment of aPKCs to maintain its active conformation. The schematic shows the canonical interaction of the PB1 domain of aPKCs with that of p62 (dotted line) and the previously undescribed interaction between the basic pseudosubstrate and acidic surface on p62. This interaction tethers the pseudosubstrate out of the substrate-binding cavity of aPKCs, allowing constitutive activity on the scaffold. The positively charged peptide ZIP competes with the pseudosubstrate for binding to the acidic surface of p62, displacing the pseudosubstrate and allowing it to re-engage in the substrate-binding site of aPKC to affect intramolecular autoinhibition of the kinase. UBA, ubiquitin-associated domain; Myr, myristoylated peptide.
