FIGURE 1.
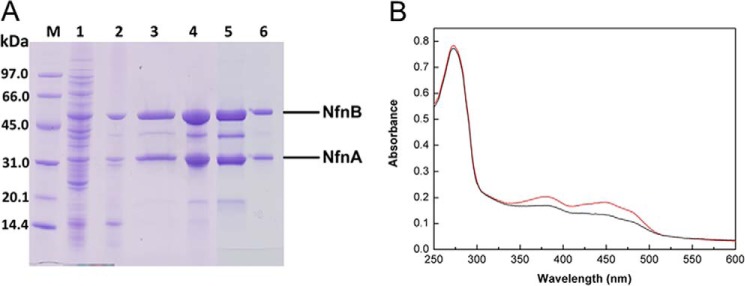
Purification of the recombinant NfnAB from T. maritima. A, SDS-PAGE. Lane M, low molecular weight SDS marker (GE Healthcare); lane 1, cell extract of recombinant E. coli cells; lane 2, supernatant after heat treatment of 80 °C for 30 min; lane 3, elution fraction of Strep-Tactin column; lane 4, NfnAB after iron-sulfur cluster reconstitution in vitro; lane 5, concentrate of elution peak A from HiPrep Sephacryl S-200; lane 6, concentrate of elution peak B from HiPrep Sephacryl S-200. After purification with the Strep-Tactin column, the preparation still contained some contaminating proteins, which turned out to be NfnB proteolysis products as shown by tandem MALDI-TOF-TOF MS analysis. B, UV-visible absorption spectra of NfnAB (1.2 mg/ml) in 100 mm Tris-HCl, pH 7.5, before and after iron-sulfur reconstitution in vitro (black and red lines). 6.5 and 9.0 mol iron per mol NfnAB was determined, respectively.
