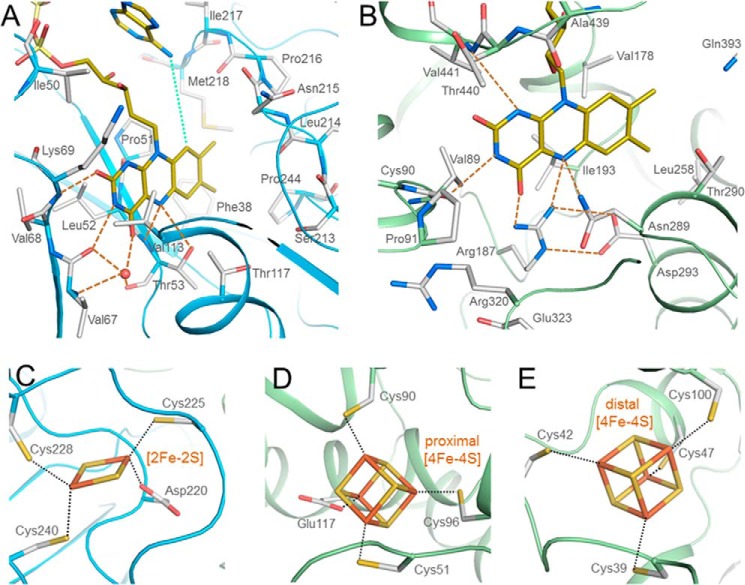
FIGURE 3.
Binding of FAD and the iron-sulfur clusters in the NfnAB complex. A, a-FAD (yellow) is attached to the β-barrel domain of NfnA (deep sky blue) in a bent conformation, the shortest distance between its N6A and C9 being 5.0 Å (green dots). Hydrogen bonds are indicated with orange dashed lines. B, b-FAD is bound in an elongated conformation at the C-terminal end of the β-sheet of the FAD domain. Arg187 directly interacts with N5 and O4 of the isoalloxazine ring that stabilize, in particular, the oxidized state. C, the [2Fe-2S] cluster is bound to NfnA. D and E, the proximal and distal [4Fe-4S] clusters are located in NfnB. The [2Fe-2S] and proximal [4Fe-4S] clusters are coordinated by unusual aspartate and glutamate ligands, respectively.