FIGURE 1.
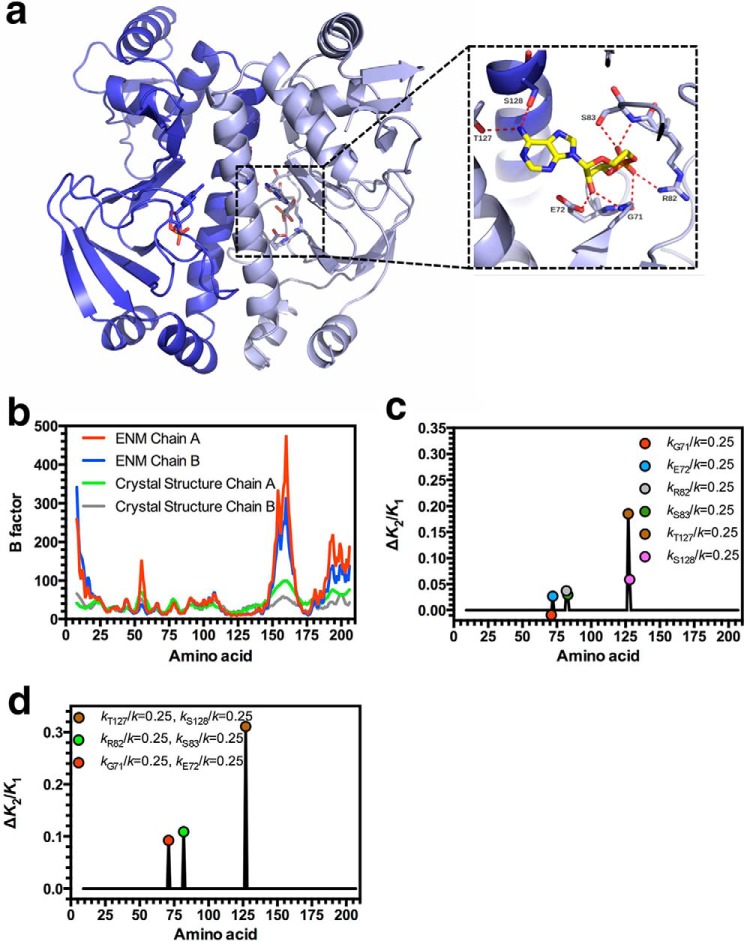
The influence of CAP-cAMP contacts on allostery in CAP. a, ribbon diagram of the x-ray crystal structure of CAP (Protein Data Bank code 4HZF) showing the secondary and tertiary structures of the CAP homodimer with cAMP bound. The inset shows the hydrogen-bonding network at the cAMP binding site in the wild type protein. The structure and inset are shown in different orientations for clarity. The labeled amino acids in the inset contact cAMP and are analyzed in this study. b, B-factor plotted against amino acid number for the crystal structure and the manually curated ENM for chain A and chain B of the CAP crystal structure. c, the change in cooperativity (K2/K1) that occurs when kR/k is varied at the indicated residue. d, the change in cooperativity (K2/K1) that occurs when kR/k is varied for pairs of indicated residues.