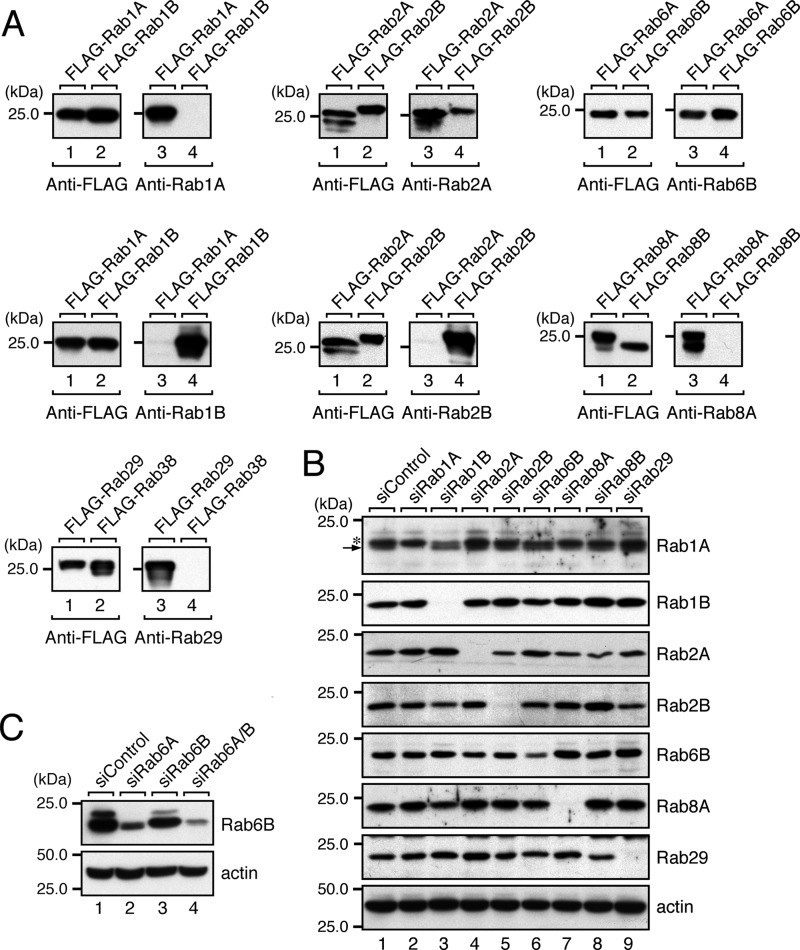
FIGURE 4.
Knockdown of candidate Rabs in HeLa-S3 cells as revealed by immunoblotting with specific antibodies. A, specificity of the antibodies used in this study. The specificity of each antibody was evaluated by using recombinant FLAG-tagged mouse Rab proteins expressed in COS-7 cells. The specificity of anti-Rab1A antibody was evaluated by analyzing COS-7 cell lysates expressing FLAG-Rab1A and FLAG-Rab1B by 10% SDS-PAGE followed by immunoblotting with HRP-conjugated anti-FLAG tag antibody (left panel) and anti-Rab1A antibody (right panel). All of the antibodies except anti-Rab2A antibody and anti-Rab6B antibody specifically recognized a single Rab isoform, whereas the anti-Rab2A antibody weakly recognized Rab2B, and the anti-Rab6B antibody recognized both Rab6A and Rab6B equally. B, knockdown of candidate Rabs in HeLa-S3 cells with specific siRNAs. Total cell lysates of HeLa-S3 cells that had been knocked down by each Rab siRNA were analyzed by 10% SDS-PAGE followed by immunoblotting with the antibodies indicated. A longer SDS-PAGE gel than usual (14 cm long instead of 8.5 cm long) was used to detect Rab1A by separating Rab1A (arrow) from the upper nonspecific bands (asterisk). All of the Rabs except Rab6B were almost completely knocked down by siRNA treatment. Because our anti-Rab6B antibody also recognized Rab6A (see A), we used partially Rab6A-knocked down samples only in the Rab6B panel (see “Experimental Procedures” for details). The residual signals in lane 6 (fifth panel) most likely correspond to endogenous Rab6A. C, double knockdown of Rab6A and Rab6B in HeLa-S3 cells with specific siRNAs. Note that knockdown of Rab6A alone dramatically decreased the anti-Rab6B immunoreactive bands without affecting the Golgi morphology (see Fig. 2A). In addition, double knockdown of Rab6A/B in HeLa-S3 cells did not further increase the rate of Golgi fragmentation (data not shown). The positions of the molecular mass markers (in kDa) are shown on the left in A–C.