Abstract
Ofatumumab is a humanized anti-CD20 monoclonal antibody that has been approved by the FDA for the treatment of patients with chronic lymphocytic leukemia. We conducted a phase II single-arm study at a single center. Patients received ofatumumab (300 mg then 1000 mg weekly for 12 weeks) and methylprednisolone (1000 mg/m2 for 3 days of each 28-day cycle). Twenty-one patients enrolled, including 29% with unfavorable cytogenetics (del17p or del11q). Ninety percent of patients received the full course without dose reductions or delays. The overall response rate was 81% (17/21) with 5% complete response, 10% nodular partial response, 67% partial response, 14% stable disease and 5% progressive disease. After a median follow-up of 31 months, the median progression-free survival was 9.9 months and the median time to next treatment was 12.1 months. The median overall survival has not yet been reached. The combination of high-dose methylprednisolone and ofatumumab is an effective and tolerable treatment regimen. This regimen may be useful for patients who are unable to tolerate more aggressive therapies, or have not responded to other treatments.
Introduction
Although the development of chemoimmunotherapy regimens has improved the overall survival of patients with chronic lymphocytic leukemia (CLL), CLL is still an incurable disease, and most patients experience relapsed disease, even after achieving complete responses (CRs) without detectable minimal residual disease (MRD).1 In the relapsed or refractory setting, many patients are unable to tolerate aggressive or myelosuppressive chemoimmunotherapy regimens owing to age, comorbidities or pre-existing marrow suppression.2, 3 Ibrutinib (Imbruvica, Pharmacyclics, Sunnyvale, CA, USA) was approved for the treatment of such patients. Response rates are high, but the CR rate is low and some patients have developed resistance owing to mutations in the drug-binding domain.4, 5
Ofatumumab (Arzerra, GlaxoSmithKline, Brentford, Middlesex, UK) is a humanized anti-CD20 monoclonal antibody approved as a single agent for the treatment of patients with relapsed or refractory CLL, or in combination with chemotherapy for patients requiring initial therapy.6 As a single agent, previous studies reported overall response rates (ORR) of 47–58%, specifically in patients who had disease refractory to fludarabine and alemtuzumab, or were not candidates for alemtuzumab therapy.7 Single-agent ofatumumab therapy infrequently achieves CRs by objective working group criteria.8
Previously, we determined that high-dose methylprednisolone (HDMP) and rituximab is a highly active regimen. Although single-agent rituximab at conventional doses is associated with a low rate of objective responses,9 rituximab in combination with HDMP produced nearly universal responses and was safe and well-tolerated.10, 11 On the basis of this rationale, a phase II single-center clinical trial was conducted to determine the activity of HDMP in combination with ofatumumab in patients with relapsed or refractory CLL.
Patients and methods
Patients
Patients with previously treated CLL, an indication for treatment defined by working group guidelines, age 18 years or greater, and performance status 0–2 according to Eastern Cooperative Oncology Group (ECOG) system were eligible for consent and enrollment.
Exclusion criteria included concurrent malignancy (excluding basal and squamous-cell skin cancers), concurrent anti-cancer therapy, expected life expectancy of <3 months, active bacterial or fungal systemic infections, or HIV or Hepatitis B or C positivity by serology. Patients predicted to be vulnerable to adverse effects of high-dose corticosteroids were also excluded, including patients with diabetes mellitus, active peptic ulcer disease, untreated metabolic disorders such as hypothyroidism and Cushing's disease, or a history of steroid-induced psychosis, pancreatitis or diverticulitis. Patients were also excluded if known to have a hypersensitivity to ofatumumab, or a history of anaphylaxis to rituximab or alemtuzumab. Subjects with current active hepatic or biliary disease (with exception of patients with Gilbert's syndrome or asymptomatic gallstones) were also excluded, as were subjects with uncontrolled autoimmune hemolytic anemia or autoimmune thrombocytopenia. Finally, patients were excluded based on the following laboratory parameters: platelet count <50 000/μl, neutrophils <1000/μl, creatinine >2.0 times upper normal limit, total bilirubin >1.5 times upper normal limit (unless a known history of Gilbert's disease), alanine aminotransferase >2.5 times upper normal limit, alkaline phosphatase >2.5 times upper normal limit.
Study design and treatment
This was an open label, nonrandomized, single institution-based clinical trial. Treatment with HDMP and ofatumumab was administered over 84 days in three consecutive 4-week (28 days) cycles. Patients received HDMP sodium succinate at 1000 mg/m2 body surface area (mg/m2) as a 90-min infusion for 3 consecutive days each 28-day cycle for a total of three cycles, on days 3–5 during cycle one and on days 1–3 of cycles two and three. Cycles 1–3 were administered without scheduled interruption every 28 days for a total of 12 weeks of therapy. Ofatumumab 300 mg was administered on day 1 of cycle 1 followed by 12-weekly doses of 1000 mg. When ofatumumab and HDMP were administered on the same day, ofatumumab infusions began after completion of the methylprednisolone infusion. There was no dose reduction of ofatumumab or HDMP; in the case of toxicity, doses were held for a maximum of 4 weeks before removal from the study.
Subjects received allopurinol 300 mg by mouth daily for 3 days before and at least during the first cycle of treatment unless they were allergic to allopurinol. Subjects received famotidine or equivalent before each dose of methylprednisolone on days 1–3. Subjects also received premedication with diphenhydramine 50 mg (or equivalent antihistamine) by mouth or by vein and acetaminophen 1000 mg by mouth before each dose of ofatumumab. On days when HDMP was not administered, subjects also received glucocorticoids (prednisolone or equivalent) up to 50 mg by vein as premedication ~30 min before ofatumumab infusions.
Prophylaxis against infections was mandated. All subjects received fluconazole 100 mg or alternative antifungal prophylaxis by mouth daily while receiving treatment and for 2 months after their last cycle. All subjects also received one tablet of trimethoprim/sulfamethoxazole DS or alternative pneumocystis prophylaxis by mouth twice a day, three times a week while receiving treatment and for 2 months after their last cycle. Subjects with a history of herpes infection or zoster received acyclovir 400 mg PO twice daily or alternative herpes virus prophylaxis while receiving treatment and for 2 months after their last cycle.
Blood glucose was measured on days of HDMP administration. For blood glucose over 201 mg/dl, glycemic control was achieved by giving regular insulin subcutaneously.
Disease status and response were assessed by history and physical and laboratory evaluation every 4 weeks during active treatment, 2 months after completion of treatment, at least every 3 months until 9 months after assessment of response, and at least every 6 months thereafter until progression or withdrawal from the study.
Efficacy
The primary end point of the study was CR based on 2008 international working group in CLL (iwCLL) guidelines.8 Responses were assessed by the principal investigator and confirmed by independent monitor. In accordance with the iwCLL guidelines, CR was determined 2 months after completion of therapy, and PR must have been maintained for >2 months. Computed tomography scans were used to confirm CRs. Secondary end points included MRD by four-color flow cytometry, ORR, progression-free survival (PFS) (starting from time from first ofatumumab infusion), overall survival, safety and tolerability and correlation with biologic prognostic factors including cytogenetics, zeta-chain-associated protein kinase 70 (ZAP-70) and immunoglobulin heavy chain (IgVH) mutations.
Safety evaluation
Severity of adverse events (AEs) was graded by investigators according to Common Terminology Criteria for Adverse Events version 4.0 (for nonhematologic AE) or iwCLL guidelines for hematologic AEs. Serious AEs were monitored and reported from the time informed consent was given until 3 years after completion of the third cycle of treatment, or until progressive disease requiring further therapy. Subjects were removed from the study for progressive disease identified by iwCLL criteria or serious AEs or reactions to ofatumumab or HDMP. Blood samples were drawn at all visits during the study period and centrally analyzed. Additional samples were drawn at local laboratories if clinically indicated, but were not used to determine clinical responses.
Statistical analysis
The study was powered to detect a significant improvement in CR rate against historical controls. A sample size of 21 patients was required to have a power of 80% (alpha of 0.05) to detect a CR rate of 20%, based on null hypothesis CR rate of 4%. Fisher's exact test univariant analysis of multiple variables was used to identify pretreatment patient characteristics associated with achieving a CR or overall response to therapy. P-values <0.05 were considered significant. Early stopping rules were based on toxicity grade and serious AEs. The trial would have stopped if four subjects experienced grade 4 nonhematologic toxicities deemed possibly related to study drug, or if there were two deaths at least possibly related to study drug.
Monitoring and study oversight
The clinical trial was approved by the University of California, San Diego Institutional Review Board and Protocol Review and Monitoring Committee. Informed consent was obtained from all patients. Accuracy and fidelity of all clinical, laboratory and safety data were reviewed and audited by an independent monitor.
Results
Between October 2010 and January 2012, we enrolled 21 patients with median age 63 years (range 46–75 years). Among them 29% (6/21) were at least 70-year-old, 29% (6/21) had a cumulative illness rating scale score >6, 29% (6/21) had unfavorable cytogenetics (del17p or del11q) and 76% (16/21) had CLL cells that expressed unmutated IgVH genes. The median number of prior therapies was three. All patients had previously received treatment with rituximab and two patients had received prior alemtuzumab therapy. In all, 85% (18/21) of patients had been treated with chemoimmunotherapy regimens and 24% (5/21) had disease courses meeting standard criteria for fludarabine resistance3 (Table 1. Patient characteristics).
Table 1. Baseline patient characteristics.
| Characteristics | No. of patients | % |
|---|---|---|
| Median age of patients in the study | ||
| > 65 | 11 | 52 |
| 65 or younger | 10 | 48 |
| Sex | ||
| Male | 18 | 86 |
| Female | 3 | 14 |
| Prior treatment | ||
| >2 prior regimens | 11 | 52 |
| 2 or fewer prior regimens | 10 | 48 |
| Prior chemoimmunotherapy | 14 | 67 |
| Prior rituximab-containing regimen | 21 | 100 |
| Fludarabine refractory | 5 | 24 |
| Rai stage at screening | ||
| 0 | 0 | 0 |
| 1 or 2 | 7 | 33 |
| 3 or 4 | 14 | 67 |
| Cytogenetics/ FISH | ||
| del17p | 4 | 19 |
| del11q | 2 | 10 |
| Trisomy 12 | 4 | 19 |
| del13q | 4 | 19 |
| Other abnormalities | 2 | 10 |
| Normal karyotype | 5 | 24 |
| ZAP-70 | ||
| Positive | 13 | 62 |
| Negative | 7 | 33 |
| No information | 1 | 5 |
| IgVH | ||
| Unmutated | 16 | 76 |
| Mutated | 5 | 24 |
| Performance status (ECOG) | ||
| 0 | 14 | 67 |
| 1-2 | 7 | 33 |
| CIRS score | ||
| > 6 | 6 | 29 |
| 6 or less | 15 | 71 |
Abbreviations: CRIS, cumulative illness rating scale; ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization; IgVH, immunoglobulin heavy chain; ZAP-70, zeta-chain-associated protein kinase 70.
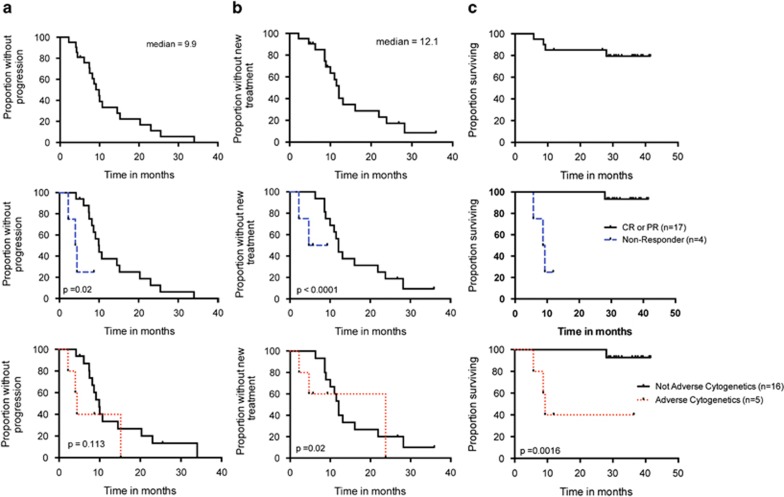
The ORR was 81% (17/21) with 5% CR (1/21), 10% nodular partial response (PR) (2/21), 67% PR (14/21), 14% s.d. (3/21) and 5% progressive disease (1/21). The patient who achieved a CR did not have detectable MRD in the bone marrow by four-color flow cytometry (<0.1% of cells). After a median follow-up of 31.0 months, the median PFS was 9.9 months (range, 2–34 months), the median treatment-free survival was 12.1 months (range, 2–36 months) (Table 2), and the median overall survival has not yet been reached (Figure 1).
Table 2. Summary of responses by iwCLL category.
| No. of patients | Percent | |
|---|---|---|
| Complete response | 1 | 4.8 |
| MRD negative | 1 | 4.8 |
| MRD positive | 0 | 0 |
| Nodular partial response | 2 | 9.5 |
| Partial response | 14 | 66.7 |
| Stable disease | 3 | 14.3 |
| Progressive disease | 1 | 4.8 |
Abbreviations:
iwCLL, international working group in chronic lymphocytic leukemia; MRD, minimal residual disease.
Figure 1.
PFS (a), Treatment-free survival (b) and overall survival (c) graphed for all patients (top row), according to response to therapy (middle row) and according to cytogenetic risk group, with Del(17p), Del(11q) or complex cytogenetics considered adverse (bottom row). Copyright: 123
We analyzed relevant clinical and biological variables. Patients who achieved a response (CR or PR) had longer survivals, as would be expected (Figure 1, middle row). In addition, patients with adverse cytogenetics (Del 17p, Del 11q or complex karyotype) had a lower response rate and shorter PFS and treatment-free survival, although the sample size was small (Figure 1, bottom row). We did not find a significant association between ORR and PFS and any other demographic or prognostic variables analyzed (other cytogenetic risk groups, age, gender, IgVH mutation, ZAP-70, Rai stage, splenomegaly >5 cm, lymph nodes >5 cm or fludarabine-refractory disease) (Table 3).
Table 3. Responses by patient characteristics.
| Characteristics/variable | OR | CI 95% | P-value |
|---|---|---|---|
| Demographics | |||
| Age (>65) | 1.1 | 0.1–9.9 | 1.0 |
| Female | 2.2 | 0.09–51 | 1.0 |
| Baseline ECOG>0 | 0.42 | 0.04–3.8 | 0.57 |
| Cytogenetics | |||
| del17p | 0.02 | 0.001–0.43 | 0.01 |
| del11q | 0.12 | 0.005–2.7 | 0.27 |
| Trisomy 12 | 3.0 | 0.1–67.3 | 0.55 |
| del13q | 3.0 | 0.1–67.3 | 0.55 |
| Prognostic/biologic | |||
| IgVH unmutated (>98%) | 0.25 | 0.01–5.5 | 0.53 |
| ZAP-70 (>20% expression) | 0.56 | 0.05–6.7 | 1.0 |
| β2-Microglobulin (>2 × ULN) | 0.42 | 0.05–3.8 | 0.57 |
| Clinical | |||
| High Rai (⩾3) | 0.61 | 0.05–7.2 | 1.0 |
| LN pretreatment >5 cm | 0.64 | 0.05–8.5 | 1.0 |
| Splenomegaly pre-tx >5cm | 5.1 | 0.2–110.3 | 0.28 |
| Fludarabine refractory | 0.92 | 0.07–11.5 | 1.0 |
| Prior regimens>2 | 0.08 | 0.004–1.7 | 0.09 |
Abbreviations:
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio.
Safety
The treatment was well-tolerated. Ninety percent of patients (19/21) received the full course without dose reductions or delays. The majority of AEs were grade 1 or 2. Nineteen percent of patients had grade 3 neutropenia and 5% had grade 3 thrombocytopenia. There were no grade 4 hematologic toxicities and no cases of grade 3 or higher anemia. Nonhematologic toxicities were graded according to CTCAE 4 guidelines. Recurring grade 3 toxicities were hyperglycemia (15 patients or 71%), nonmelanoma skin cancer or precursor skin lesions requiring surgical intervention (4 patients or 19%). Other grade 3 toxicities included a single episode each of pneumonia, acute coronary syndrome, atrial fibrillation, renal calculi and hypocalcemia. Although insomnia, anxiety, fatigue and infusion reactions were common, they did not result in any grade 3 or 4 toxicities (Tables 4a and 4b). Deaths occurred during study follow-up for four patients. One patient died of complications following allogeneic stem cell transplant and three patients died from progressive CLL.
Table 4a. Nonhematologic adverse events.
| Adverse Events | Total (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| Hyperglycemia | 20 (95%) | — | 10 (48%) | 10 (48%) | — |
| Insomnia | 16 (76%) | 14 (67%) | 2 (10%) | — | — |
| Fatigue | 12 (57%) | 8 (38%) | 4 (19%) | — | — |
| Infusion reaction | 11 (52%) | 5 (24%) | 6 (29%) | — | — |
| Rash | 11 (52%) | 9 (43%) | 2 (10%) | — | — |
| Upper respiration infection | 11 (52%) | 4 (19%) | 7 (33%) | — | — |
| Hypokalemia | 9 (43%) | 8 (38%) | 1 (5%) | — | — |
| Anxiety | 8 (38%) | 7 (33%) | 1 (5%) | — | — |
| Arrhythmia | 7 (33%) | 5 (24%) | 1 (5%) | 1 (5%) | — |
| Elevated ALT (SGPT) | 7 (33%) | 6 (29%) | 1 (5%) | — | — |
| Hypocalcemia | 7 (33%) | 5 (24%) | 1 (5%) | 1 (5%) | — |
| Abdominal pain | 6 (29%) | 5 (24%) | 1 (5%) | — | — |
| Back pain | 6 (29%) | 5 (24%) | 1 (5%) | — | — |
| Allergic rhinitis | 4 (19%) | 2 (10%) | 2 (10%) | — | — |
| Hyperkalemia | 3 (14%) | 2 (10%) | 1 (5%) | — | — |
| Weakness | 3 (14%) | 2 (10%) | 1 (5%) | — | — |
| Weight gain | 3 (14%) | 2 (10%) | 1 (5%) | — | — |
| Cough | 2 (10%) | 1 (5%) | 1 (5%) | — | — |
| Hyperhidrosis | 2 (10%) | 1 (5%) | 1 (5%) | — | — |
| Pneumonia | 1 (5%) | — | — | 1 (5%) | — |
| Influenza | 1 (5%) | — | — | 1 (5%) | — |
| Kidney stone | 1 (5%) | — | — | 1 (5%) | — |
| Myocardial infarction | 1 (5%) | — | — | 1 (5%) | — |
| Squamous cell skin cancer | 1 (5%) | — | — | 1 (5%) | — |
Abbreviation: ALT, alanine aminotransferase; SGPT, serum glutamate pyruvate transaminase.
Table 4b. Hematologic adverse events.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
|---|---|---|---|---|---|
| Neutropenia | 0 | 4 (19%) | 3 (14%) | 3 (14%) | 10 (48%) |
| Anemia | 9 (43%) | 1 (5%) | 0 | 0 | 10 (48%) |
| Thrombocytopenia | 7 (33%) | 1 (5%) | 0 | 0 | 8 (38%) |
Discussion
In this clinical trial, we observed that the combination of HDMP and ofatumumab is well-tolerated and highly active for the treatment of patients with relapsed or refractory CLL. Both the clinical effect and the safety profile compare favorably with previously published regimens in the relapsed or refractory setting. The AEs mainly reflected anticipated symptoms of high-dose steroids, such as hyperglycemia, anxiety and insomnia. Importantly, serious infections were not encountered, although the importance and mandated use of prophylactic antibacterial, antiviral and antifungal medications should not be understated. Moreover, the infrequency of grade 4 myelotoxicity makes this regimen particularly attractive for the treatment of patients who are not able to tolerate more toxic chemotherapy-based regimens.
The ORR of 81% was higher than that achieved with single-agent ofatumumab in other published studies. For example, the ORR was 50% in patients with fludarabine and alemtuzumab refractory or ineligible patients7 and only 4.1% in the ofatumumab arm of the RESONATE trial for patients with relapsed or refractory CLL.12 Even though this study was not designed to make such comparisons directly, the results presented here are encouraging. Moreover, our study population had high-risk clinical and biological features, including predominantly male patients or patients with leukemic cells with high levels of ZAP-70 (62%) or unmutated IgVH genes (76%). This study also included many patients who would be considered heavily pretreated, with the majority of patients having had prior chemoimmunotherapy based treatment. Also of note, all patients in this study previously received and progressed following rituximab therapy.
It can also be noted that HDMP and ofatumumab appear to result in a response rate comparable to the emerging tyrosine kinase inhibitors like Ibrutinib, with comparable tolerability and with potential advantages including treatment cost.4, 13
Potential weaknesses of this study include the modest sample size, which was initially designed to determine the CR rate of HDMP/ofatumumab compared with historical controls using the HDMP-therapy backbone.10, 11 The CR rate of 5% did not meet the primary end point of this clinical study. With the majority of patients achieving a PR, correlative laboratory studies to determine factors associated with nonresponse were not feasible either. Univariant analysis with a variety of biological and clinical characteristics was statistically significant for del17p or adverse cytogenetics, although the strength of this correlation is lessened by the sample size as well.
The addition of HDMP allowed for the use of lower ofatumumab doses compared with the standard dosage and schedule of 2000 mg for 12 doses. In addition, the standard regimen gives the final four doses of ofatumumab monthly, for a total treatment duration of 6 months, whereas the duration of treatment in this clinical trial was 3 months only. The high response rate in this study therefore further supports the synergistic activity of anti-CD20 antibodies with HDMP as we have previously reported using rituximab.10, 11 The decreased dose and duration of treatment with ofatumumab in this regimen may also contribute to its feasibility and affordability. It is also possible that the relatively brief PFS (although still longer than previously reported with single-agent ofatumumab) could have been improved further by additional monthly maintenance of ofatumumab doses.
Finally, the outcome from this trial is consistent with the previous data reported by our group showing favorable safety and efficacy using HDMP in combination with rituximab. The CR rate and PFS were higher with the rituximab-containing regimen; although this study was not designed to make that comparison directly, patient characteristics may have contributed to this result. Regardless, HDMP appears to improve the activity of the monoclonal antibody. We noted a higher response rate with the combination than previously reported with treatment with the antibody alone. This supports additional clinical trials of this approach with other monoclonal antibodies, such as Gazyva (obinutuzumab, Genentech, South San Francisco, CA, USA).14
In summary, HDMP and ofatumumab is a well-tolerated regimen despite the presence of comorbid conditions in high-risk CLL patients. In addition, this combination is associated with a high response rate that can be achieved within 3 months of initiation of therapy. As both agents are clinically approved and readily available, this regimen constitutes a valid alternative for patients with relapsed or refractory CLL.
Acknowledgments
This work was supported by GlaxoSmithKline for clinical trial costs and drug supply. Additional support was provided by NIH 5P01CA081534-14 (TJK and JEC), Lymphoma Research Foundation Grant 285871 (JEC) and the Tower Cancer Research Foundation (MYC). We thank Laura Rassenti, Monica Spydell, Sheila Hoff, Jenny Perales and Andrew Greaves for their assistance with data management. Funding support was provided by GlaxoSmithKline for clinical trial procedures and drug supply, but not for direct salary support. GlaxoSmithKline had no role in the design of the study, collection or analysis of data or decision to publish.
The authors declare no conflict of interest.
References
- 1Kipps TJ. Chronic lymphocytic leukemia and related disordersKaushansky K, Beutler E, Kipps T, Seligsohn U, Prchal J Williams Hematology. New York: McGraw-Hill, 2010. p 1431–1482. [Google Scholar]
- 2Brown JR. The treatment of relapsed refractory chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 2011; 2011: 110–118. [DOI] [PubMed] [Google Scholar]
- 3Keating MJ, O'Brien S, Kontoyiannis D, Plunkett W, Koller C, Beran M et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma 2002; 43: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 4Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA et al. Targeting BTK with Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014; 370: 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Shanafelt T, Lanasa MC, Call TG, Beaven AW, Leis JF, Laplant B et al. Ofatumumab-based chemoimmunotherapy is effective and well tolerated in patients with previously untreated chronic lymphocytic leukemia (CLL). Cancer 2013; 119: 3788–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Wierda WG, Kipps TJ, Mayer J, Stilgenbauer S, Williams CD, Hellmann A et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on chronic lymphocytic leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9O'Brien SM, Kantarjian H, Thomas DA, Giles FJ, Freireich EJ, Cortes J et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 2001; 19: 2165–2170. [DOI] [PubMed] [Google Scholar]
- 10Castro JE, Sandoval-Sus JD, Bole J, Rassenti L, Kipps TJ. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia 2008; 22: 2048–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Castro JE, James DF, Sandoval-Sus JD, Jain S, Bole J, Rassenti L et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia 2009; 23: 1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Byrd JC, Brown JR, O'Brien SM, Barrientos JC, Kay NE, Reddy NM et al. Randomized comparison of ibrutinib versus ofatumumab in relapsed or refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma: Results from the phase III RESONATE trial. J Clin Oncol 2014; 32: 5s. [Google Scholar]
- 13Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013; 31: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Salles G, Morschhauser F, Lamy T, Milpied N, Thieblemont C, Tilly H et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood 2012; 119: 5126–5132. [DOI] [PubMed] [Google Scholar]