Abstract
AIM: To observe effects of ACOL on fibrinogen (FIB), fibrin degrading products (FDP) and changes of FIB and FDP concentration in rabbits with intro-abdominal exudates during 7 d after major abdominal surgery.
METHODS: Sixty New Zealand rabbits were randomly divided into 4 groups: ACOL group, the control group, DCT group and the normal group. After being modeled, except the normal group, the other 3 groups were treated with different ways for a week; the intro-abdominal exudates of rabbits in the 4 groups were drawn for FIB and FDP measurement once daily during 7 d after major abdominal surgery.
RESULTS: FIB and FDP in the intro-abdominal exudates altered in a regular way and ACOL could change the concentration of FIB and FDP in the intra-abdominal exudates after major abdominal surgery.
CONCLUSION: ACOL can prevent intestinal adhesion by reducing the concentration of FIB and raising that of FDP in the intro-abdominal exudates after major abdominal surgery.
INTRODUCTION
With a high incidence of 90%[1-2], intra-abdominal adhesions are the most common complications after major abdominal surgery, and overall 70% patients of intra-abdominal adhesion have the history of previous laparotomies[3]. The exact mechanisms about this complication have widely been explored. The damage of peritoneum and its base during laparotomies leads to intra-abdominal traumatic inflammation, and thus the normal intra-abdominal physical condition is destroyed, inflammatory cells and medias are released. Coagulation, kinin peptide, fibrinolysis, arachidonic acid (AA) participate in this process. The activity of plasminoge activator (t-PA), matrix metalloproteinase (MMP) and their inhibitor changes accordingly[4-8]. The related factors of adhesion, such as TNF-α, TGF-β, and VEG-F, are involved in regulations of the whole process[9-11], which results in the abnormally higher concentration of free FIB and FDP in the intra-abdominal exudates. The disturbance of the intra-abdominal homeostasis among secreting, dissolving and absorbing together with over-deposition of fibrin in the abdominal cavity finally leads to fibrous adhesion[12]. In this research, occurrence and development of intra-abdominal adhesions will be discussed by observing the dynamical changes of FIB and FDP in rabbits’ intra-abdominal exudes continuously during 7 d postoperatively. Antiadhesion concentrated oral liquid (ACOL) was used as soon as possible to improve the intra-abdominal physical condition and help to regain the homeostasis of internal environments to prevent the formation of the fibrous jelly in the abdominal cavity. This experiment provided evidence for the punctual and strategic prevention and treatment of the postoperative intra-abdominal adhesion.
MATERIALS AND METHODS
Experimental animal
Sixty healthy New Zealand rabbits of both sexes, weighing from 2.5-3.5 kg, were purchased from Laboratory Animal Center, Medical College of Xi’an Jiaotong University.
Drugs, reagents and instruments
ACOL consisted of Rubarb, Cotex Magnoliae Officinalis, Fructus Auvantii Immature, Caucklandiu Lappal, Folium Raphani Radish Seed, Radix Aucklandiae, Radix Astragali, Cortex Cinnamomi, Fructu Amomi, Radix Angelicae Cinensis, Rhizoma Zingiberis and Radix Salvia Miltiorrhiza. Da-Cheng-Qi Tang (DCT) consisted of Rubarb, Ctex Magnoliae Officinalis, Fructus Aurantii Immature and Natrli Sulfas. Both were provided by Shaanxi College of TCM, and decocted with water and concentrated through reflux with distillatory and water bath to extract the decoction containing 1.0 g crude/mL. FIB test kit was purchased from Changzheng Medical Scientific Limited Company, Shanghai, (batch number f4900704). FDP test kit was purchased from Institute of Microbiology and Epidemiology, Academy of Military Medical Sciences, (batch number 900325). Automatic Biochemical Analyzer 7170 type was from Hitachi, Tokyo, Japan.
Animal models
The experiment was carried out in the clean but not sterile condition under 30 g/L procaine anesthesia (0.5 mL/kg, iv). After shaving and skin-disinfecting, the laparoscopy was performed through a 4 cm vertical midline incision to enable the following performances: (1) appendectomy, (2) perforation of an incision (1 cm in diameters) at 5 cm off pylorus on the lesser curvature with punching device, then repair of the perforation, (3) 2 × 2 cm2 area of the cecal parietal peritoneum being carefully stripped, (4) injection of the solution of Talc powder 1mL/kg and 5 mL distilled water into the abdominal cavity prior to skin closure, (5) splenectomy, and (6) leaving a tube for drainage in the spleen pit because all the animals were fasted for 8 h after operation. The study protocol was in accordance with the guideline for animal research and was approved by the Research Committee of the hospital.
Grouping and medication
The 60 New Zealand rabbits were randomly divided into 4 groups: normal group, ACOL group, control group and DCT group, with 15 rabbits in each group. From the second day postoperation, rabbits in ACOL group were administered ACOL (5 mL/kg) gastrogavage twice daily, the DCT group was administered DCT, the control group was given normal saline (NS) instead, while the normal group had none treatment. The medication was carried out from the second day of modeling and lasted for 1 wk. 15 mL NS was injected through the drainage cube into the abdominal cavity of each model animal once daily, and 5 min later the abdominal exudates was drawn for FDP and FIB detection.
Statistical analysis
All data were expressed as mean ± SD, and statistical analysis were performed by Student-Newman-Keuls test, ANOVA analysis, Difference between groups were compared by SNK-q test, with P < 0.05 considered to have statistical significance.
RESULTS
Concentration curves of FIB and FDP in rabbits abdominal exudates during the 7 d postoperatively
The concentration curve of FIB in the rabbits’ intra-abdominal exudates showed an increasing trend in the initial 5 d, and reached its peak on the 5th d. After that, it showed a decreasing trend, while on the 7th d still above the preoperative value (0.1 ± 0.017) g/L. (Figure 1). At the same time, the concentration curve of FDP in the exudates showed increase in the first 4 d, arriving at its peak on the 3rd or the 4th d. After that, it showed decrease, while the value of the 7th d was also still above the preoperative value (0.3 ± 0.012) μg/mL. (Figure 2). In comparison with the concentration curve of FIB and FDP in the exudates during the 7 d postoperatively, the DCT group was similar to the ACOL group while the normal group similar to the control group. (Figure 1, Figure 2). In comparison with the average concentration of FIB and FDP in intra-abdominal exudates during 7 d postoperatively, there was remarkable difference among the DCT group, normal group, and control group (P < 0.05). The ACOL group showed a remarkable difference (P < 0.05), compared with the other three groups. (Table 1, Table 2).
Figure 1.
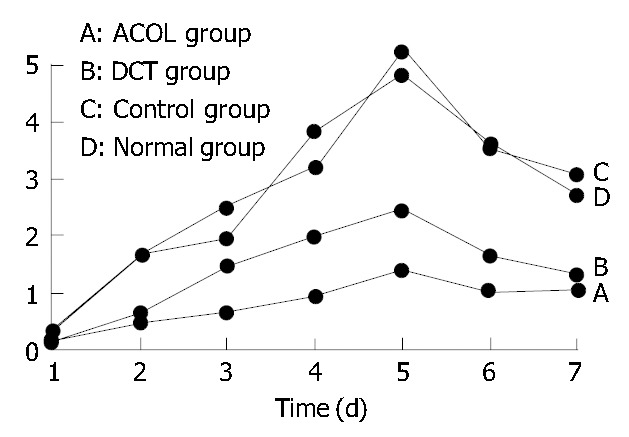
Concentration variety of FIB in healthy rabbits’ abdominal cavity during 7 d after operation.
Figure 2.
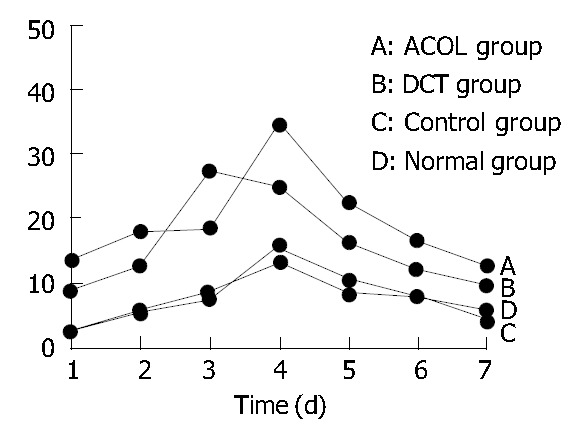
Concentration variety of FDP in healthy rabbits’ abdominal cavity during 7 d after operation.
Table 1.
Concentration of FIB and FDP in healthy rabbits’ abdominal cavity during 7 d after operation
| Group | d 1 | d 2 | d 3 | d 4 | d 5 | d 6 | d 7 |
| FIB (g/L) | |||||||
| Normal group | 0.236 ± 0.052 | 1.621 ± 0.083 | 1.927 ± 0.121 | 3.813 ± 0.154 | 4.823 ± 0.183 | 3.612 ± 0.076 | 2.721 ± 0.055 |
| Control group | 0.27 ± 0.071 | 1.587 ± 0.012 | 2.471 ± 0.134 | 3.224 ± 0.126 | 5.256 ± 0.113 | 3.533 ± 0.054 | 3.081 ± 0.046 |
| DCT group | 0.145 ± 0.021a | 0.652 ± 0.046a | 1.432 ± 0.061a | 1.961 ± 0.051a | 2.431 ± 0.112a | 1.621 ± 0.107a | 1.321 ± 0.062a |
| ACOL group | 0.121 ± 0.032a | 0.457 ± 0.054a | 0.628 ± 0.052a | 0.927 ± 0.072a | 1.367 ± 0.077a | 1.024 ± 0.068a | 1.026 ± 0.059 |
| FDP (μg/mL) | |||||||
| Normal group | 2.328 ± 0.072 | 5.821 ± 0.176 | 8.368 ± 0.372 | 13.472 ± 0.961 | 8.462 ± 0.371 | 7.721 ± 0.262 | 5.642 ± 0.078 |
| Control group | 2.452 ± 0.077 | 5.262 ± 0.132 | 7.526 ± 0.327 | 15.641 ± 0.625 | 10.431 ± 0.362 | 7.651 ± 0.241 | 4.326 ± 0.062 |
| DCT group | 8.675 ± 0.323a | 12.638 ± 0.672a | 27.236 ± 2.331a | 24.638 ± 2.725a | 16.369 ± 1.026a | 12.445 ± 0.328a | 9.652 ± 0.375a |
| ACOL group | 13.372 ± 0.541a | 19.338 ± 1.321a | 17.328 ± 1.124a | 34.325 ± 4.68a | 22.438 ± 1.372a | 16.367 ± 0.554a | 12.771 ± 0.366 |
P < 0.05 vs other groups.
Table 2.
Average concentration of FIB and FDP in healthy rabbits’ abdominal cavity during 7 d after operation
| Normal group | Control group | DCT group | ACOL group | |
| Concentration of FIB (g/L) | 2.679 ± 0.096 | 2.760 ± 0.079 | 1.36 ± 60.066a | 0.793 ± 0.059a |
| Concentration of FDB (μg/mL) | 7.442 ± 0.268 | 7.613 ± 0.261 | 15.947 ± 01.121a | 19.424 ± 1.417a |
P < 0.05 vs the normal group and the control group.
Analyses of the average concentration of FIB and FDP in rabbits abdominal exudates
Compared with the control group and the normal group, FIB and FDP in the DCT group showed a significant difference (P < 0.05); while in comparison with the other 3 groups, those of the ACOL group showed a significant difference (P < 0.05).
DISCUSSION
So far, there is no safe and effective prophylaxis available for intra-abdominal adhesions. Scientists try to find the answer from studying the exact mechanism of intra-abdominal adhesions, thus various experiments have been conducted to elaborate the exact mechanism of intra-abdominal adhesions and its prevention. The theory of traumatic inflammation is prevalent in elucidating the mechanism of intra-abdominal adhesions[14]. Actually, multiple factors are involved in the development of postoperative intra-abdominal adhesions, such as operative injury, tissue ischemia and foreign materials left during operation[14-15]. Any damage to the peritoneum and its base can lead to the release of inflammatory exudates, which contained FIB that causes fibrous adhesion and eventually results in the following two consequences: First, the fibrin is eliminated through the phagocytosis and endogenous fibrinolysis system, and the regeneration of mesothelial cell will cover the wound surface thus to get it repaired physiologically. Second, the wound is not covered by the regenerated mesothelial cells and no physiological repair occurs. Instead, fibrous adheres to other serosa in 12-24 h after operation, and then fibroblasts forms in place of the fibrous matrix, which causes the production of the fibrous collagen and eventually leads to fibrous adhesion. The adhesion can be absorbed completely if mesothelial cells are intact, but when the mesothelial cells are seriously destroyed, its plasminogen activator activity (PAA) is depressed, and with the depression value to that low as 60% or less, the fibrous adhesion will form as a consequence[16-19].
In this experiment, the postoperative concentration levels of FIB and FDP in the rabbits’ intra-abdominal exudates were constantly observed during the postoperative period of 7 d. The values of both FIB and FDP in intra-abdominal exudates increased considerably after 24 h postoperatively, arrived at its peak on the 4th or 5th d after operation, and then started to decrease till the 7th d, but still not being able to recover to the its normal preoperative level. The whole process is rather similar to the traumatic inflammation process. Giving the experimental animals some drugs which had been proved effective in promoting the ability of intestinal movement, the incidence of intestinal adhesion decreased remarkably, which indicates that the intestinal adhesion is related to traumatic inflammation, intestinal movement, and intra-abdominal physical conditions.
Under normal condition, there is a little serous fluid in the peritoneal cavity, which, on one hand, is necessary to keep the surface of the viscera moist and reduce the friction among the viscera, and on the other hand, functions to help recover the viscera and defend it against damage. Normally, the omentum, parietal and visceral peritoneum keeps a balance between releasing and absorbing serous fluid in the peritoneal cavity. Maintenance of this balance depends upon the normal sequential movement of intestines and the unobstructed movement of intestinal contents. After abdominal surgery, ileuses paralysis and the retention of the intestinal contents can effect the normal sequential movement of intestines and the smooth movement of intestinal contents. With intestines paralyzed postoperation, the contents are retained in the intestinal cavity. Endotoxin and bacteria in the stool are absorbed from intestines, which results in the abnormally high level endotoxin in the blood, which, in turn, stimulates intestines and worsens their paralysis, thus weakening the intestines’ sequential movement and obstructing the smooth movement of their contents. Inflammatory reaction is indispensable to the healing of gastrointestinal and peritoneal damage in operation. Tissue injury, local hemorrhage, cell inactivity and coagulation will call for the inflammation response, which originates from the following vascular reaction: the inflammatory mediums such as histamine, 5-HT, bradykinin, and prostaglandin. cause small blood vessels to first contract transiently, then dilate with congestion, and thus increase the permeability of blood vessels, which enables water, electrolyte, serum proteins, neutrophils and monocytes to enter tissue inter-space, so traumatic inflammatory reaction happens to help heal the damage. The FIB that has permeated into the wound gaps can turn into fibrin and fill the gaps to function as the network for cells to proliferate. However these inflammatory exudes can also enter peritoneal cavity from the wound, and the homeostasis of intra-abdominal serous postoperation is destroyed. During the damage, the serous secretion is far beyond the absorbing capability of peritoneal cavity. At the same time, high concentration of FIB in the wound exudes increases the concentration of FIB in peritoneal cavity. With the weakened movement of intestines in the early stage of postoperation and the temporarily destroyed normal physical condition, the exudes among viscera containing higher concentration of FIB easily constitute early fibrous adhesion, which is although still fragile at its early stage. Such traumatic inflammatory response arrives at the climax around 48 to 72 h postoperation, then disappears gradually.The early fragile adhesion can turn into tight one through endometrial stage and mould stage. We can see that the early recovery of the intestinal movement and abdominal physical condition postoperation is vital for prevention of the intestinal adhesion[20-23].
The present study also indicates the traumatic inflammation caused by major abdominal surgery can cause the release of cytokines, the subsequent effects on endothelial cells, inflammatory cells and mesothelini cells, which produce and release plasminogen activator activity inhibitor I or II (PAI1, PAI2). Previous research showed TNF-α, IL-I, TGFβ, alone or combined, can stimulate the cultured mesothelini cells to produce plasminogen activator activity inhibitor I or II (PAI1and PAI2), which ultimately causes the disturbance of homeostasis between FIB and FDP intra-abdominal exudes. Consequently fibrin depositing on the surface of intra-abdominal viscera constitutes adhesion[24-27].
In essence, intestinal adhesion is that the normal physical condition of peritoneum and the homeostasis between secreting and absorbing sera is destroyed, with higher FIB concentration in the peritoneal cavity, the weakened sequential movement of intestines and the dysfunctional movement of the intestinal contents, so that the wound healing appears among intestine, peritoneal and other viscera. The incidence of intestinal adhesion is related to the degree of the completeness of the peritoneal mesothelial cells in that when mesothelial cells are destroyed seriously, the fibrous adhesion forms. The damage of peritoneal mesothlium cannot be avoided in laparoscopy no matter how gentle the operation is. Since intestinal adhesions postoperation are almost inevitable to some extent[28], recovery of the physical condition in abdominal cavity after operation as soon as possible can reduce the incidence and the adventure of intestinal adhesion[29].
Now we can see, how to shorten the time of traumatic inflammation caused by major abdominal surgery, how to recover the normal physical condition of intra-peritoneum, and how to recover the homeostasis between FIB and FDP, is the vital to prevent the intra-abdominal adhesion postoperatively. The pharmaceutics of laxative remedis represented by Rhubarb start with purgation of intestinal contents, improve the early recovery of intestinal movement as soon as possible. The pharmacologic research of Rhubarb develops quickly, clinic application is wider and wider. The main components of Rhubarb include: Anthraquinones (emodin, rhein, aloe emdin), Polysacchavide from Rheum palmatum (DHP-1, DHP-2) Tannins (hydrolysates type, condensed type), Modern pharmacology finds the effective component in Rhubarb of purgation is Anthraquinone, which effects on colon and increases the tension of the middle and far parts of colon to make them move faster, while no affection on the function of small bowels to absorb the nutritions. experiments reported: if vasectomy the part between colon and small bowels, then inject Anthraquinone into small bowels, but the medicine affects on the colon. Emodin combines with the muscle albumen of affected organ, then behavior cholineroic reaction, which can excite the M receiptors of intestinal smooth muscle, increasing intestinal movement, inhibiting Na+ K+ ATP enzyme in intestinal cell membrane. It also can hinder the absorption and transportion of Na+, increase osmotic pressure of intra-intestines, keep lots of water, promote intestinal movement to defecate[30]. On one hand through relieving the temporary enterogenic bacterial translocation caused by intestine paralyzed. Rhubarb prevent intestines from ischemia-reperfusion injury, protect gut barrier function, lower the level of endotoxin in blood to demodulate the releasion of cytokine and inflammatory mediums intra-peritoneum. on the other hand, it can lower the ability of endotoxin in blood which stimulates the target cells such as mesothelial cell, platelet et al, Lower the sensitivity of these target cells to the stimulis of endotoxin in blood, make the injurys caused by cytokine, inflammatory mediums easy to be controled. It also can reduce the level of PGE and CAMP in cerebrolate. Lots of experiments prove: Rhubarb remarkably inhibits the functions of cytokine secreted by Macrophage cells which are stimulated by endotoxin. emodin strongly inhibits proliferations of T lymphocyte. Perhaps it is realized through reducing the expression of inflammatory mediums IL-2mRNA and concentration of Ca2+ in cell membrane[31]. emodin has excellent Antagonism to early inflammatory exudes, increasing penetration of microvascular, and leucocytes migration[32]. In the condition of injures, such as tissue ischemia, endometrial injury, leucocytes Infiltration, and platelet aggregation, lots of free radicals are brought out, which cause pathologic lipidoxidation and induce producation of cytotoxinic lipid free radical and lipid peroxides malondiadehyde (MDA). Rhubarb can inhibit the producation of MDA, relieve the injure of tissue and organ. Rhubarb is a kind of strongly free radical scavengers, and an inhibitor of lipidoxidation, it has function to remove most of free radicals[33]. Rhubarb can remove O2, H2O2 and other peroxides, inhibit lipid oxidation, Rhubarb themselves on pharmacology have the function of immune regulate two-wayly. emodin improve Indraft of Ca2+ outside of leucocytes, this function have some relations with improving immune of leucocytes; while Polysacchavide from Rheum palmatum inhibits Indraft of Ca2+ outside of leucocytes and releasion of Ca2+ inside of leucocytes, and the digree of inhibitation have some relations with its dosage, so for leucocytes it has inhibit function[34], Rhubarb can inhibits the producation of red blood corpuscles antibody, and inhibit the function of T cell, improve phagocytosis of macrophage, it is benefit to regulate immunation. It also can inhibits synthetic of germ’s protein and nucleic acid, ACOL adds some medicine such as Radix Astragali, Radix Salvia Miltiorrhiza, Rhizoman Zingiberis to DCT. Those functions lie in invigorating qi and strengthening asthenia, activating blood to resolve stagnation, and improving the whole condition of patiences postoperatively. They also can enhance the resistance of human being, improve the microcirculation. It is benefits to the recovery of the normal physical condition of intra-peritoneum postoperatively. Altogather, ACOL can suppress bacterial and anti-inflammation, protect organs, improve microcirculation through exclude from accumulating. It relieves the accumulation of excrement and bacterial through improving intestinal movement, so it can lower temporary high level toxin in blood, reduce the release and producation of histamine, bradiykinin, 5-HT, prostaglandin, et al, improve the perfusion of blood, relieve the traumitic imflammation intra-peritoneum postoperatively, recover the homeostasis between FIB and FDP as soon as possible, reduce the incidence of adhesion intra-peritoneum.
Footnotes
Edited by Zhang JZ and Xu FM
References
- 1.Scott-Coombes DM, Whawell SA, Thompson JN. The operative peritoneal fibrinolytic response to abdominal operation. Eur J Surg. 1995;161:395–399. [PubMed] [Google Scholar]
- 2.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–273. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 3.Parker MC, Ellis H, Moran BJ, Thompson JN, Wilson MS, Menzies D, McGuire A, Lower AM, Hawthorn RJ, O'Briena F, et al. Postoperative adhesions: ten-year follow-up of 12, 584 patients undergoing lower abdominal surgery. Dis Colon Rectum. 2001;44:822–829; discussion 829-830. doi: 10.1007/BF02234701. [DOI] [PubMed] [Google Scholar]
- 4.Edelstam G, Lecander I, Larsson B, Astedt B. Fibrinolysis in the peritoneal fluid during adhesions, endometriosis and ongoing pelvic inflammatory disease. Inflammation. 1998;22:341–351. doi: 10.1023/a:1022322814288. [DOI] [PubMed] [Google Scholar]
- 5.Saed GM, Diamond MP. Modulation of the expression of tissue plasminogen activator and its inhibitor by hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2003;79:164–168. doi: 10.1016/s0015-0282(02)04557-0. [DOI] [PubMed] [Google Scholar]
- 6.Hellebrekers BW, Trimbos-Kemper GC, Bakkum EA, Trimbos JB, Declerck PJ, Kooistra T, Emeis JJ. Short-term effect of surgical trauma on rat peritoneal fibrinolytic activity and its role in adhesion formation. Thromb Haemost. 2000;84:876–881. [PubMed] [Google Scholar]
- 7.Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763–768. doi: 10.1016/s0015-0282(00)01799-4. [DOI] [PubMed] [Google Scholar]
- 8.Chegini N, Zhao Y, Kotseos K, Ma C, Bennett B, Diamond MP, Holmdahl L, Skinner K. Differential expression of matrix metalloproteinase and tissue inhibitor of MMP in serosal tissue of intraperitoneal organs and adhesions. BJOG. 2002;109:1041–1049. doi: 10.1111/j.1471-0528.2002.01334.x. [DOI] [PubMed] [Google Scholar]
- 9.Chegini N, Kotseos K, Zhao Y, Bennett B, McLean FW, Diamond MP, Holmdahl L, Burns J. Differential expression of TGF-beta1 and TGF-beta3 in serosal tissues of human intraperitoneal organs and peritoneal adhesions. Hum Reprod. 2001;16:1291–1300. doi: 10.1093/humrep/16.6.1291. [DOI] [PubMed] [Google Scholar]
- 10.Zeng J, Li XF. Molecular mechanism and medicinal prevention and treatment of peritoneal adhesions. World J Digestol. 2003;11:1429–1432. [Google Scholar]
- 11.Diamond MP, El-Hammady E, Wang R, Saed G. Regulation of transforming growth factor-beta, type III collagen, and fibronectin by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Fertil Steril. 2003;79:1161–1167. doi: 10.1016/s0015-0282(03)00075-x. [DOI] [PubMed] [Google Scholar]
- 12.Liu JY, Zeng GX, Zheng YB. Observe the peritoneal constitution morphology during the development of wounded intestine adhesion. Chine J Sterol Image Anal. 2003;8:97–101. [Google Scholar]
- 13.Treutner KH, Schumpelick V. [Prevention of adhesions. Wish and reality] Chirurg. 2000;71:510–517. doi: 10.1007/s001040050848. [DOI] [PubMed] [Google Scholar]
- 14.Chegini N. Peritoneal molecular environment, adhesion formation and clinical implication. Front Biosci. 2002;7:e91–115. doi: 10.2741/A911. [DOI] [PubMed] [Google Scholar]
- 15.Duron JJ, Olivier L, Khosrovani C, Gineste D, Jost JL, Keilani K. [Natural history of postoperative intraperitoneal adhesions. Surely, a question of the day] J Chir (Paris) 1993;130:385–390. [PubMed] [Google Scholar]
- 16.Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76:560–567. doi: 10.1016/s0015-0282(01)01964-1. [DOI] [PubMed] [Google Scholar]
- 17.Lai HS, Chen Y, Chang KJ, Chen WJ. Effects of octreotide on epidermal growth factor receptor, tissue plasminogen activator, and plasminogen activator inhibitor during intraperitoneal adhesion formation. J Gastroenterol. 2003;38:555–560. doi: 10.1007/s00535-002-1103-6. [DOI] [PubMed] [Google Scholar]
- 18.Molinas CR, Elkelani O, Campo R, Luttun A, Carmeliet P, Koninckx PR. Role of the plasminogen system in basal adhesion formation and carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80:184–192. doi: 10.1016/s0015-0282(03)00496-5. [DOI] [PubMed] [Google Scholar]
- 19.Witz CA, Thomas MR, Montoya-Rodriguez IA, Nair AS, Centonze VE, Schenken RS. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Steril. 2001;75:385–390. doi: 10.1016/s0015-0282(00)01699-x. [DOI] [PubMed] [Google Scholar]
- 20.Alatas E, Günal O, Alatas O, Colak O. Octreotide prevents postoperative adhesion formation by suppressing peritoneal myeloperoxidase activity. Hepatogastroenterology. 2000;47:1034–1036. [PubMed] [Google Scholar]
- 21.Haney AF. Identification of macrophages at the site of peritoneal injury: evidence supporting a direct role for peritoneal macrophages in healing injured peritoneum. Fertil Steril. 2000;73:988–995. doi: 10.1016/s0015-0282(00)00490-8. [DOI] [PubMed] [Google Scholar]
- 22.Toh H, Torisu M, Shimura H, Kitsuki H, Uchiyama A, Itoh H, Ohsato K. In vitro analysis of peritoneal adhesions in peritonitis. J Surg Res. 1996;61:250–255. doi: 10.1006/jsre.1996.0112. [DOI] [PubMed] [Google Scholar]
- 23.Vural B, Cantürk NZ, Esen N, Solakoglu S, Cantürk Z, Kirkali G, Sökmensüer C. The role of neutrophils in the formation of peritoneal adhesions. Hum Reprod. 1999;14:49–54. doi: 10.1093/humrep/14.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Cheong YC, Shelton JB, Laird SM, Richmond M, Kudesia G, Li TC, Ledger WL. IL-1, IL-6 and TNF-alpha concentrations in the peritoneal fluid of women with pelvic adhesions. Hum Reprod. 2002;17:69–75. doi: 10.1093/humrep/17.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Lindholt JS, Jørgensen B, Shi GP, Henneberg EW. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2003;25:546–551. doi: 10.1053/ejvs.2002.1872. [DOI] [PubMed] [Google Scholar]
- 26.Falk K, Björquist P, Strömqvist M, Holmdahl L. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Br J Surg. 2001;88:286–289. doi: 10.1046/j.1365-2168.2001.01647.x. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Y, Li H, Bunn C, Bartold PM. The expression of plasminogen activator system in a rat model of periodontal wound healing. J Periodontol. 2001;72:849–857. doi: 10.1902/jop.2001.72.7.849. [DOI] [PubMed] [Google Scholar]
- 28.Wang XC, Gui CQ, Zheng QS. Combined therapy of allantoin, metronidazole, dexamethasone on the prevention of intra-abdominal adhesion in dogs and its quantitative analysis. World J Gastroenterol. 2003;9:568–571. doi: 10.3748/wjg.v9.i3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricketts SA, Sibbons PD, Green CJ. Quantitative analysis of the development of experimentally induced post surgical adhesions: a microstereological study. Int J Exp Pathol. 1999;80:325–334. doi: 10.1046/j.1365-2613.1999.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo WX. The pharmacologic research of Rhubarb. J Jianghan University. 2002:30; 60–61. [Google Scholar]
- 31.Kuo YC, Meng HC, Tsai WJ. Regulation of cell proliferation, inflammatory cytokine production and calcium mobilization in primary human T lymphocytes by emodin from Polygonum hypoleucum Ohwi. Inflamm Res. 2001;50:73–82. doi: 10.1007/s000110050727. [DOI] [PubMed] [Google Scholar]
- 32.Qi H. Anti-inflammation of emodine. Zhongcaoyao. 1999;30:550–552. [Google Scholar]
- 33.Matsuda H, Morikawa T, Toguchida I, Park JY, Harima S, Yoshikawa M. Antioxidant constituents from rhubarb: structural requirements of stilbenes for the activity and structures of two new anthraquinone glucosides. Bioorg Med Chem. 2001;9:41–50. doi: 10.1016/s0968-0896(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen D, Qiao L, Jing B. [Effect of rhubarb on oxygen radicals leakage from mitochondria of intestinal mucosa in burned rats] Zhongguo Zhongxiyi Jiehe Zazhi. 2000;20:849–852. [PubMed] [Google Scholar]


