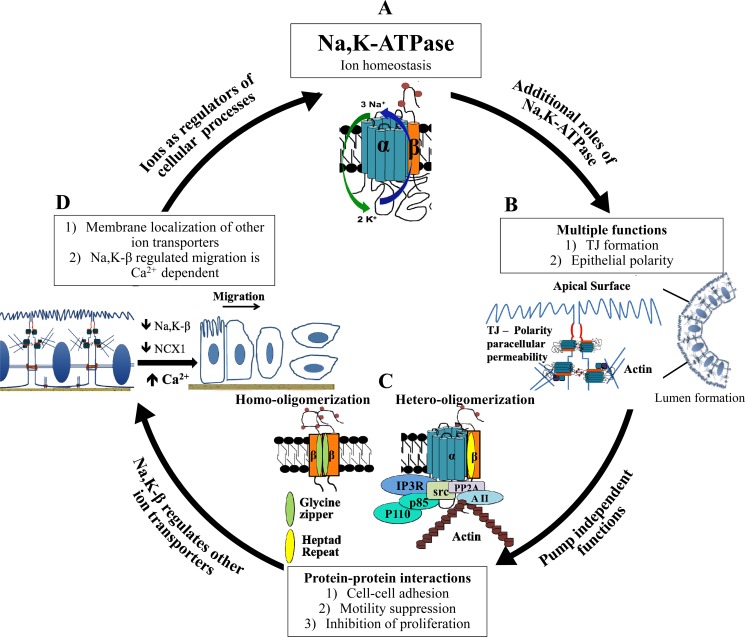
Fig. 1.
Role of Na-K-ATPase in ion-dependent regulation of epithelial cell functions. A: since its discovery by Jens Skou in 1957 (51), the role of Na-K-ATPase (composed of Na-K-α and Na-K-β subunits) in the regulation of ion homeostasis has been studied extensively. B: studies in the early 2000s elucidated other functions of Na-K-ATPase in the formation and maintenance of tight junctions (TJ) and preservation of epithelial polarity. A pictorial representation shows Na-K-ATPase localized to TJ regulating paracellular permeability and the formation of fluid-filled lumen. C: in the mid to late 2000s, various groups studied the pump-independent roles of Na-K-ATPase, specifically, the role of protein-protein interactions in the regulation of cell-cell adhesion and in suppression of motility and proliferation. The cartoon depicts protein-protein interactions mediated by Na-K-ATPase subunits. The glycine zipper motif (shown in green in the homodimerization cartoon) and N-glycan domains (shown as 3 red circles in the extracellular region) of Na-K-β mediate the interaction of Na-K-β with Na-K-β involved in cell adhesion. The heptad repeat motif of Na-K-β that mediates the interaction of Na-K-α with Na-K-β is shown in orange in the heterodimerization (Na-K-α/β) cartoon. Na-K-α interaction with Src, inositol 1,4,5-trisphosphate receptor (IP3R), and p85 subunit of phosphatidylinositol 3-kinase (PI3K) is shown, along with Na-K-β association with annexin II (A II) and protein phosphatase 2A (PP2A). D: additional functions of Na-K-β in the regulation of membrane localization of other ion transporters [such as Na/Ca exchanger 1 (NCX1), large-conductance Ca2+-activated K+ (BKCa) channel, and Na-K-2Cl cotransporter isoform 2 (NKCC2)], apart from its cognate partner Na-K-α, have come to light in recent years. Recently, we showed that Na-K-β-mediated migration is Ca2+-dependent and the loss of Na-K-β mediates reduction in NCX1, thereby increasing intracellular Ca2+ concentration ([Ca2+]i) and activating PI3K/ERK. In conclusion, ions are the mediators of Na-K-ATPase-dependent cellular processes governing formation and maintenance of the epithelial phenotype. The cartoon represents the increase in the rate of migration of epithelial cells with Na-K-β knockdown, mediated by a reduction of NCX1 and an increase in intracellular Ca2+.