Abstract
Phase-amplitude coupling of two pacemaker activities of the small intestine, the omnipresent slow wave activity generated by interstitial cells of Cajal of the myenteric plexus (ICC-MP) and the stimulus-dependent rhythmic transient depolarizations generated by ICC of the deep muscular plexus (ICC-DMP), was recently hypothesized to underlie the orchestration of the segmentation motor pattern. The aim of the present study was to increase our understanding of phase-amplitude coupling through modeling. In particular the importance of propagation velocity of the ICC-DMP component was investigated. The outcome of the modeling was compared with motor patterns recorded from the rat or mouse intestine from which propagation velocities within the different patterns were measured. The results show that the classical segmentation motor pattern occurs when the ICC-DMP component has a low propagation velocity (<0.05 cm/s). When the ICC-DMP component has a propagation velocity in the same order of magnitude as that of the slow wave activity (∼1 cm/s), cluster type propulsive activity occurs which is in fact the dominant propulsive activity of the intestine. Hence, the only difference between the generation of propagating cluster contractions and the Cannon-type segmentation motor pattern is the propagation velocity of the low-frequency component, the rhythmic transient depolarizations originating from the ICC-DMP. Importantly, the proposed mechanism explains why both motor patterns have distinct rhythmic waxing and waning of the amplitude of contractions. The hypothesis is brought forward that the velocity is modulated by neural regulation of gap junction conductance within the ICC-DMP network.
Keywords: coupled oscillators, ICC, interstitial cells of Cajal, intestinal motility, phase-amplitude coupling
the motor patterns of the small intestine are dependent on, or influenced by, smooth muscle cell properties, pacemaker cells, the central and peripheral nervous systems, hormones, and other blood-borne substances. The role of pacemaker activities generated by interstitial cells of Cajal was the focus of the present study. The ICC associated with the myenteric plexus (ICC-MP) generate electrical oscillatory activity, the “slow waves,” the dominant omnipresent pacemaker activity of the small intestine, that propagates into the musculature, providing it with cyclic changes in excitability. This determines the rhythmic transient nature of the contractions; it determines the frequency and propagation characteristics of the slow wave controlled rhythmic motor activity. Slow-wave-driven peristaltic activity can be demonstrated convincingly when comparing wild-type mice with WWv mutant mice that lack functional ICC-MP. When barium was gavaged into the stomach of wild-type mice, the motor pattern of the proximal small intestine, upon arrival of barium, showed rhythmic propulsive activity at the slow wave frequency (13). These slow-wave-driven contractions were lumen occluding and strongly propulsive (13). Using high-resolution manometry in a segment of human ileum in vitro (30), the slow-wave-driven contractions were of lesser amplitude, referred to as ripples, and the direction of propagation was quite variable, rendering this motor pattern less propulsive under such conditions. The slow-wave-driven contractions can also occur in clusters, which can appear stationary (15) or can be propagating (15, 52). When propagating, the contractions can be powerful, rendering the clustered contractions forcefully propulsive (30). The clusters often appear in a rhythmic manner, at a much lower frequency than the contractions within the cluster (28, 30).
After a meal, the dominant motor pattern ought not to be propulsive, and Cannon (7) gave in 1902 the classical description of the segmentation motor pattern of the intestine; its rhythmicity was shown a few years later by Alvarez (1) to be myogenic in origin. Recently it was shown that the segmentation motor pattern of the small intestine is associated with rhythmic waxing and waning of the amplitude of the slow wave activity, as well as rhythmic waxing and waning of the contraction amplitude (25). Evidence was provided that waxing and waning developed when low-frequency rhythmic transient depolarizations originating from interstitial cells of Cajal associated with the deep muscular plexus (ICC-DMP) interacted with the omnipresent slow wave activity originating from the interstitial cells associated with the myenteric plexus (ICC-MP) through phase-amplitude coupling. That is, the phase of the low-frequency activity modulated the amplitude of the higher frequency slow wave activity, similar to interaction of brain activities at different frequencies (8). Hence these interacting myogenic electrical activities occurring in the musculature in between the two pacemaker networks were seen to be a critical part of the mechanism underlying the segmentation motor pattern. The emergence of a segmentation motor pattern appeared to be associated with the induction of the second pacemaker originating from the ICC-DMP (25). The ICC-DMP pacemaker occurs at a much lower frequency; the inductor can be a fatty acid such as decanoic acid or a neurotransmitter such as Substance P (25). The existence of a second myogenic pacemaker has been supported by studies from Jimenez and coworkers (34, 35, 41) since 2001. The segmentation motor pattern occurs in response to nutrients, as shown by the effect of decanoic acid to whole rat intestine in vitro (25), intraluminal decanoic acid or amino acids in the guinea pig in vitro (19, 20), or oleic acid in vivo, in dogs (15).
Segmentation, as with all motor patterns, has neural excitation as an important component of modulation (19). The mechanism underlying the rhythmicity of segmentation has also been proposed to originate within the neural circuitry (9, 19). The objective of the present study was to provide further insight into the role of the myogenic control system in shaping the motor patterns of the small intestine. The two pacemaker activities, the slow waves and the rhythmic transient depolarizations, were modeled as two sine waves of different frequency and their interactions were studied using phase-amplitude coupling. Particular attention was paid to the influence of the propagation velocity of the ICC-DMP component on the motor patterns.
METHODS
Electrophysiology.
All research and handling of animals was approved by the Animal Research Ethics Board at McMaster University in accordance with the standards set by the Canadian Council on Animal Care (CCAC). Female adult CD-1 mice (Charles River Laboratories International, Wilmington, MA) ∼14 wk of age were euthanized by cervical dislocation. Small segments of the jejunum (1–2 cm) were prepared for intracellular recording. The mesenteric fat was carefully cut off with fine point scissors, and the intestine was subsequently opened along the length of the mesenteric border and pinned flat on a Sylgard gel dish, mucosa facing upwards. Intracellular electrical activity was recorded from individual circular smooth muscle cells within a muscle preparation as previously described (39). In short, activity was recorded by impalement with borosilicate glass micropipettes (fire polished; length 7 cm, OD 1.5 mm, ID 0.86 mm) filled with 3 M KCl and fabricated to yield resistance between 30 and 70 MΩ, using a MultiClamp 700B amplifier (Axon Instruments, Molecular Devices, Sunnyvale, CA). Signals were digitized at an acquisition rate of 2 kHz using a Digidata 1322A acquisition system (Axon Instruments).
Tissue preparations were kept in oxygenated (95% O2-5% CO2) Krebs solution (118.1 mM NaCl, 1.0 mM NaH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 4.8 mM KCl, 11.1 mM glucose, 25 mM NaHCO3). All drugs and reagents were dissolved in deionized water except for decanoic acid [to induce the waxing and waning electrical activity (39)], which was dissolved in dimethyl sulfoxide (DMSO) and nicardipine (to reduce contractile activity to allow electrical intracellular recording), which was dissolved in 50% ethanol. DMSO never exceeded 0.1% total volume during experimentation.
For continuous wavelet transformation (CWT) analysis, ∼4 min per sample of continuous uninterrupted intracellular recordings was reduced to a sampling rate of 200 Hz and imported into Matlab (MathWorks, Natick, MA). The voltage time parameters were then analyzed by the software to produce an end product including frequency power spectrum, and a two-dimensional frequency over time contour map.
Modeling.
f(x,t) is the spatiotemporal map, a function of distance along the length of the intestine (x) and time (t). f(x,t) was modeled as the sum of two components, an ICC-DMP component fdmp(x,t) and an ICC-MP component fmp(x,t).
| (1) |
The ICC-DMP component was a sine wave of amplitude Admp (arbitrary units), angular frequency ωdmp (radians/s), and velocity vdmp (cm/s).
| (2) |
The ICC-MP component (the omnipresent slow wave) was similarly defined except that 1) the amplitude Amp was a function of the ICC-DMP component's phase ϕdmp (the “phase-amplitude coupling function”), and 2) the midpoint amplitude of the sine wave was adjusted according to α. The latter allowed the envelope of the ICC-MP component to be changed from symmetric about zero (α = 0) to asymmetric away from zero (α = nonzero).
| (3) |
The phase-amplitude coupling function Amp(ϕdmp) was a sum of a constant B (“baseline”) and a Gaussian wrapped around the [0,2π] interval (see Fig. 1K). The wrapping made sure amplitudes at 0 and 2π were equal (when the Gaussian is not centered on π) and so prevented discontinuities in fmp. The Gaussian had a height of h, standard deviation of w (radians), and center of c (radians).
| (4) |
Fig. 1.
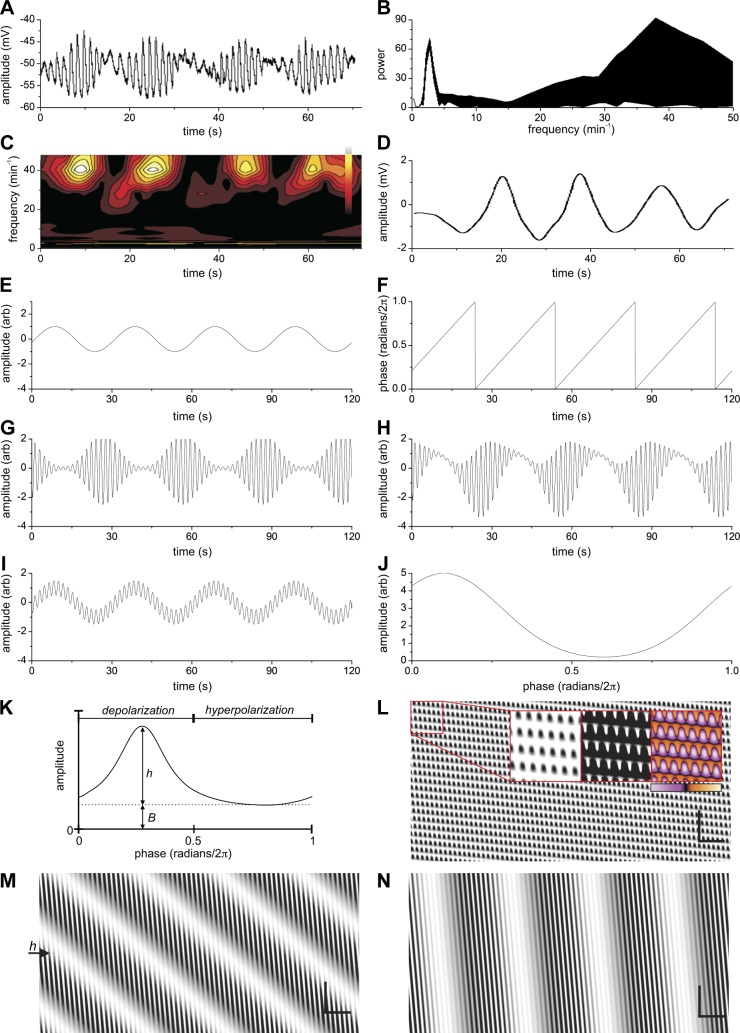
Phase-amplitude coupling of two pacemaker activities: influence of propagation velocity. A: intracellular electrical activity in the mouse small intestine showing waxing and waning of the slow wave amplitude. B and C: continuous wavelet transform of A showing a high- and low-frequency component. Frequency spectra at all times (B) and frequency-time plot (C). D: low pass filtered signal of A (8-pole Bessel 0.1-Hz cutoff). E and F: model with parameters (except as indicated below): Admp = 2; ωdmp = 2 min−1; vdmp = 0.08 cm/s; α = 0; ωmp = 34 min−1; vmp = 1 cm/s; B = 0; h = 5; c = 0.1; w = 0.18. E: the DMP component (at x = 2.5 cm). F: phase of the DMP component (at x = 2.5 cm). G: the MP component with its amplitude modulated by the phase of the DMP component through phase-amplitude coupling. H: sum of the DMP and the modulated MP component. The phase-amplitude coupling function parameters were adjusted to have the output mimic A. I: sum of DMP and MP components without phase-amplitude coupling (B = 1; h = 0). J: phase-amplitude coupling function arbitrarily modified from G to better mimic the actual recording as shown in A (see methods and Figs. 2 and 1S). K: the phase-amplitude coupling function (see methods). B = baseline, h = height; the first half is correlated to the depolarization phase of the ICC-DMP component and the second half to the hyperpolarization phase. L–N: spatiotemporal map of summed components with vdmp = 0.008 cm/s, 0.08 cm/s, and 0.8 cm/s, respectively. Note: the profiles of the amplitude over time at any one point are the same for L–N and are as shown in H. Insets in L: All three insets are from the same data. The applied contrast using black and white may suggest abrupt changes between contraction and relaxation in the segmentation pattern, but this is not the case. The color image shows the gradual changes and the Supplemental Movie S1 shows the exact changes over time. Bars in L, M, and N: horizontal: 10 s; vertical 1 cm.
The phase of the ICC-DMP component, ϕdmp (radians), was calculated as
| (5) |
Note that by this definition ϕ = 0 and 2π are the troughs of the ICC-DMP sine wave, so that the [0,π] and [π,2π] intervals correspond to depolarization and hyperpolarization, respectively. This is shifted by 0.5π from the trigonometric convention, but is more physiologically pertinent. All parameters with radian units are given in the main text divided by 2π, so that the coupling function Gaussian parameters (w,c) are normalized to the range between 0 and 1. Angular frequencies (ωdmp, ωmp) are divided by 2π/60 so they appear as per minute frequencies. The model was designed as plug-ins into ImageJ.
Calculation of phase-amplitude function from electrophysiology data.
Electrophysiology time series were bandpass filtered (8-pole Bessel) into an ICC-MP (band = 20–60 cpm) and an ICC-DMP (band = 1–6 cpm) components. For each component the analytic signal was calculated by FFT (36) and this was used to calculate either the instantaneous phase (ICC-DMP) or amplitude envelope (ICC-MP) and thereby the phase-amplitude relationship. Phase was defined by the same convention as the model.
Motility video recording and spatiotemporal mapping.
The hypothesis that underlies this manuscript came from analyzing numerous whole intestine motility video experiments both at Wuhan University and McMaster University and having numerous discussions with the coauthors of this paper both in China and Canada. For this reason, studies have been included from work in Canada (on mice) and China (on rats), also to show that the phenomena are not species specific.
Studies at McMaster University.
CD-1 mice (n = 17 female, 14 wk old) were euthanized by chloroform overdose followed by exsanguination. All animal procedures were approved by the McMaster University Animal Research Ethics Board. Lengths of small intestine were placed in organ baths containing oxygenated Krebs at 36°C and were imaged with web cameras (Macally, Toronto, Canada) mounted above the organ-bath, at a resolution of 0.014 cm/pixel at 30 Hz. Specific conditions (pressurization, drugs added to bath) varied with experiment and are indicated in figure legends.
Motility maps were created from web camera recordings with a custom plugin for ImageJ (NIH, Bethesda, MD) written by Sean Parsons. With the length of the intestine parallel to the x-axis of the camera frame, the plugin calculates the intestine's width at each pixel along the x-axis, by intensity thresholding. This gives an image of intestine width (image intensity) as a function of time (x-axis) and length along the intestine (y-axis). This image is called a “motility map” or “spatiotemporal map.” Lighter colors in the map indicate dilation and darker colors indicate contraction.
Studies at Wuhan University.
The proximal small intestine was examined from 15 adult male Sprague-Dawley rats weighing 150–300 g. Protocols were approved by the ethics committee of Renmin Hospital of Wuhan University (NSFC-81170249). Animals were killed by cervical dislocation. Krebs solution consisted of (in mM) 118.1 NaCl, 4.8 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgCl2, 12.2 glucose, and 2.5 CaCl2. The contents of the small intestine were gently flushed out using warmed Krebs solution and the external connective tissue was removed. The distal and proximal ends were cannulated and fixed to the bottom of the organ bath. The inflow tube at the proximal end was connected to a reservoir (a 50-ml syringe) placed 15 cm above the level of the small intestine with PBS (phosphate buffered solution with 10 mM indomethacin, without glucose). The outflow tube (inner diameter 3 mm, outer diameter 4 mm) was positioned in a narrow upright container (measuring cylinder of 25 ml) filled with PBS. The fluid level in the container determined the intraluminal pressure and could be adjusted by raising or lowering the fluid level. Data acquisition occurred through a Microsoft camera using Microsoft Lifecam software at a frame rate of 25/s. Video recordings were analyzed using ImageJ custom plugins as described above.
RESULTS
Modeling phase-amplitude coupling.
A program was developed to explore interaction of electrical signals that operate within the musculature of the intestine. The omnipresent slow wave activity originating from ICC-MP was represented by a sine wave at a frequency between 30 and 45 cpm. The rhythmic transient depolarizations proposed to originate from the ICC-DMP (25) were represented by a sine wave between 2 and 4 cpm. The amplitude of the ICC-MP wave was determined by the phase of the ICC-DMP wave according to a phase-amplitude coupling function for which we chose a single Gaussian (see methods for details) so that the ICC-MP amplitude peaked at a single phase of the ICC-DMP cycle (55). The ICC-DMP wave, and the ICC-MP wave modulated by the ICC-DMP, were added together (55) to produce the output signal shown in all figures and movies.1
Obtaining model parameters from intracellular recording of the electrical activity.
Intracellular electrical recordings were obtained from the circular muscle cells close to the deep muscular plexus of the small intestine in the presence of decanoic acid as reported previously (39) (Figs. 1A and 2A). The amplitude of the slow waves in the presence of decanoic acid was waxing and waning; CWT analysis revealed a broad band of high frequencies centering on 40 cpm and a low-frequency component at 3 cpm (Fig. 1, B and C). The low-frequency component in the recording of Fig. 1A was obtained by band pass filtering between 1 and 6 cpm and shown in Fig. 1D, considered the rhythmic transient depolarizations originating in ICC-DMP (25, 39).
Fig. 2.
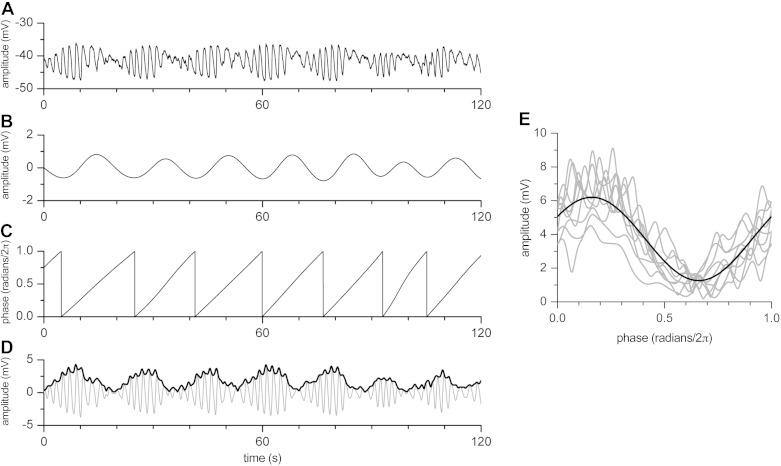
Deriving the phase-amplitude coupling function from the raw electrical data. A: intracellular electrical recording in the presence of decanoic acid. B: low-frequency (ICC-DMP) component obtained by bandpass filtering (1–4 cpm) of signal in A. C: change of phase over time from low-frequency signal in B. D: high-frequency (ICC-MP) component obtained by bandpass filtering (20–40 cpm) of signal in A (gray) and its amplitude envelope (heavy black). E: amplitude envelope of the high-frequency component, multiplied by two, plotted against the phase of the low-frequency component, i.e., the phase-amplitude coupling function. Sine fit of the data is shown in heavy black. It is interesting that the peak is shifted to the left compared with a symmetrical Gaussian function; note similarity with Fig. 1J.
The specifics of decanoic acid-induced waxing and waning patterns were used in the model: phase-amplitude coupling of two sine waves such that the phase of the low-frequency wave modulated the amplitude of the high-frequency slow wave. When this is applied using the high- and low-frequency components of Fig. 1A, the model shows a symmetrical waxing and waning pattern (Fig. 1G). However, the waxing and waning pattern is rarely perfectly symmetrical. A more realistic pattern was observed (Fig. 1H) when the center of the phase-amplitude coupling function was moved to the left (Fig. 1J). It is important to note that simple addition of the two sine waves did not result in any resemblance of the electrical recording (Fig. 1I). The interpretation is that phase-amplitude coupling of the two ICC pacemaker activities mimics the actual recording whereas simple addition of the signals does not.
To investigate the true phase-amplitude coupling function, the function was extracted from the raw electrical data in randomly chosen experiments (Fig. 2). The amplitude envelope of the high-frequency component, multiplied by two (see Fig. 2), was plotted against the phase of the low-frequency component, creating the phase-amplitude coupling function. Note that the peak of the function is shifted to the left compared with a symmetrical Gaussian function, similar to the function in Fig. 1J, which was obtained by trial and error to mimic the original recording. This means that the maximum amplitude reduction of the ICC-MP slow wave falls along the upstroke phase of the ICC-DMP rhythmic transient depolarization (see also Fig. 1K).
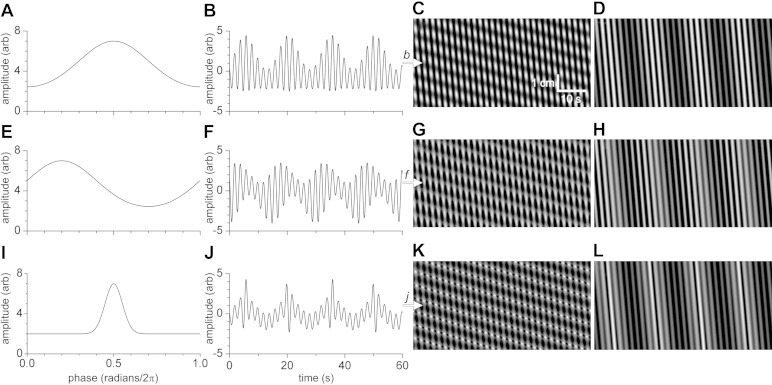
The model gives further insight into the influence of changing the parameters of the phase-amplitude coupling function for both segmentation and propulsive cluster contractions (explained below). Figure 3B shows the “Gaussian” shape of the waxing and waning with the standard phase-amplitude coupling function; when the peak is off-center to the left (i.e., ICC-MP amplitude wanes as the ICC-DMP reach maximum depolarization; Figs. 1J, 2E, and 3E), the shape of the waxing and waning is close to what is most often seen in the electrical recordings (38, 39). When the width of the coupling function is narrowed, the difference between low- and high-amplitude oscillations becomes higher (Fig. 3J), and fewer oscillations may reach mechanical threshold. The physiological regulation of the coupling function warrants further investigation.
Fig. 3.
Changing the phase-amplitude function in the model. The basic parameters for C, G, K were: ICC-DMP component, frequency 4/min, velocity 0.05 cm/min; ICC-MP component, frequency 30/min, velocity 2 cm/s. The basic parameters for D, H, L were the same except the velocity of the ICC-DMP component was 1.5 cm/s. A: phase-amplitude function. The phase-amplitude function parameters were: base 2, height 5, peak center (radians/2π) 0.5, peak width (radians/2π) 0.2. B: amplitude profile over time from C and D (at b, white arrow). C: spatiotemporal map using amplitude function of A, with ICC-DMP component at 0.05 cm/min. Please see Supplemental Movie S1 for a comprehensive understanding of the segmentation motor pattern. D: spatiotemporal map using amplitude function of A, with ICC-DMP component at 1.5 cm/min. E: phase-amplitude function. The phase-amplitude function parameters were: base 2, height 5, peak center (radians/2π) 0.2, peak width (radians/2π) 0.2. F: amplitude profile over time from G and H (at f, white arrow). G: spatiotemporal map using amplitude function of E, with ICC-DMP component at 0.05 cm/min. H: spatiotemporal map using amplitude function of E with ICC-DMP component at 1.5 cm/min. I: phase-amplitude function. The phase-amplitude function parameters were base 2, height 5, peak center (radians/2π) 0.5, peak width (radians/2π) 0.05. J: amplitude profile over time from K and L (at j, white arrow). K: spatiotemporal map using amplitude function of I, with ICC-DMP component at 0.05 cm/min. L: spatiotemporal map using amplitude function of I, with ICC-DMP component at 1.5 cm/min.
The change of a propagating contraction pattern into a segmentation motor pattern.
When slow wave activity is undisturbed (i.e., the phase-amplitude function is flat), a continuous wave of depolarization travels across the intestine at about 1 cm/s, allowing a continuing propulsive wave of contraction in its wake if the musculature is simultaneously and sufficiently excited (Fig. 4, B and F). To visualize this, amplitude profiles were constructed along the intestine and their progression over time shown in a movie (Supplemental Movie S2; Supplemental material is available with the online version of this article). The amplitudes of the contractions (decreases in intestinal diameter) were of equal value along the intestine, and the contractions propagated along the intestine (Fig. 4, F and H, and Supplemental Movie S2).
Fig. 4.
Characteristics of the mouse segmentation motor pattern. A: spatiotemporal map showing the segmentation motor pattern with an occasional propulsive contraction. B: spatiotemporal map showing a transition from segmentation to propulsion. Note that the “propagation velocity” can be calculated at the right side of the image, but is also still “visible” at the left side of the image, allowing the hypothesis that in both instances a propagating slow wave is part of the control mechanism. C: at the white arrow (c) in A, it can be seen that during segmentation, multiple contractions are identifiable at the same time along the intestine. D: at the white arrow (d) in B, it can be seen that in this section of the intestine, two propulsive contractions occur at the same time at any point of the intestine. E: model with parameters: Admp = 2; ωdmp = 8 min−1; vdmp = 0.07 cm/s; α = 0; ωmp = 50 min−1; vmp = 0.85 cm/s; B = 2; h = 5; c = 0.5; w = 0.1. F: model with same parameters as E but with no modulating DMP component (Admp = 0; B = 7; h = 0). G: at the white arrow (g) in E, it is shown that ∼7 (segmental) contractions occur at any point in time. H: at the white arrow (h) in F, it is shown that 3.5 (propulsive) contractions occur at any point in time. I: between white arrowheads (i) in E, an on-off on-off pattern of contractions is seen as shown here. Between white arrowheads in F a continuous contraction is propagating along the intestine (see also Supplemental Movies S1 and S2).
When, in the model, the propagation velocity of the slow wave was set at 1 cm/s and the second pacemaker, the rhythmic transient depolarizations, was introduced with a propagation velocity at 0.08 cm/s, the spatiotemporal map showed the typical Cannon-type segmentation activity (Figs. 1L, 4E, and 5C) (25). When the changes in intestinal diameter were shown over time in a segment of the intestine, it was clear that the indentations (the transient circular muscle contractions) did not propagate, hence a true segmentation activity promoting absorption and inhibiting propulsion (this is strikingly illustrated in Supplemental Movie S1). Hence, after phase-amplitude coupling of the slow wave with the second pacemaker, the regular propulsion is completely disturbed, and the continuous wave of depolarization has been transformed into a rhythmic wave of on-off-on-off depolarization, such that the associated contraction pattern consists of transient, abrupt, effectively nonpropagating contractions (Supplemental Movie S1). Without the second pacemaker component, the slow wave activity as modeled in Fig. 4F results in 3.5 contractions of constant amplitude traveling the intestine at any one time (Fig. 4H); when the segmentation motor pattern is induced by the second pacemaker, instead of just 3.5 indentations at any one time, now >7 indentations occur, contracting and relaxing to provide effective mixing (Fig. 4G, Supplemental Movie S1).
Fig. 5.
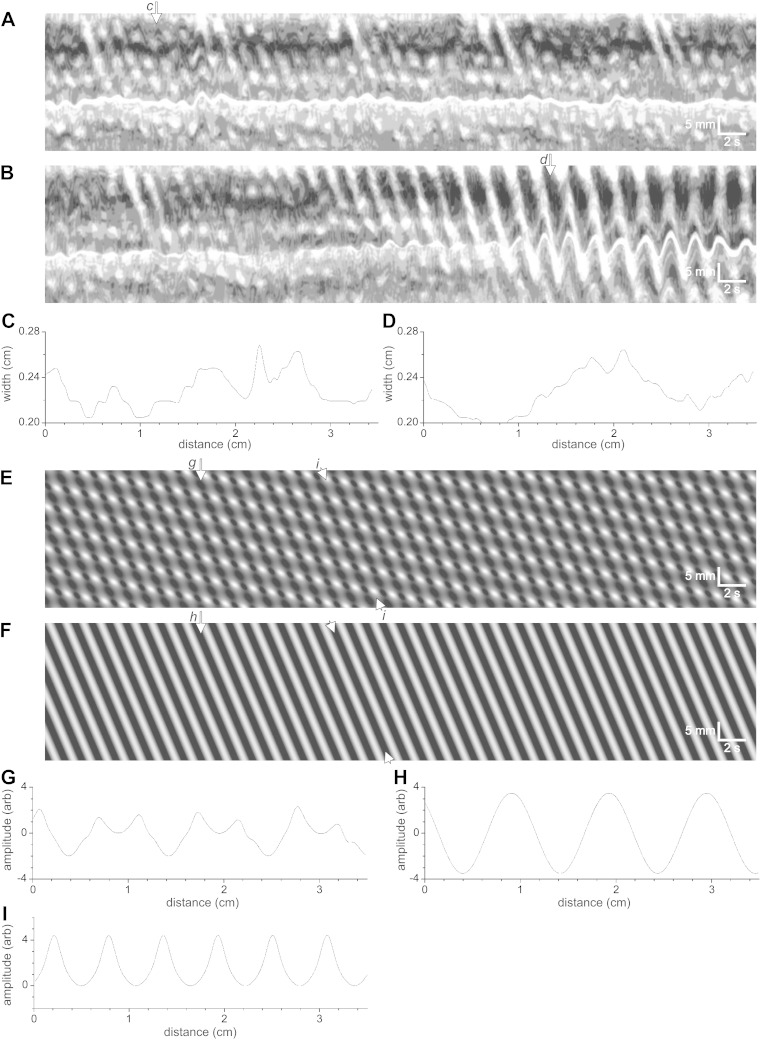
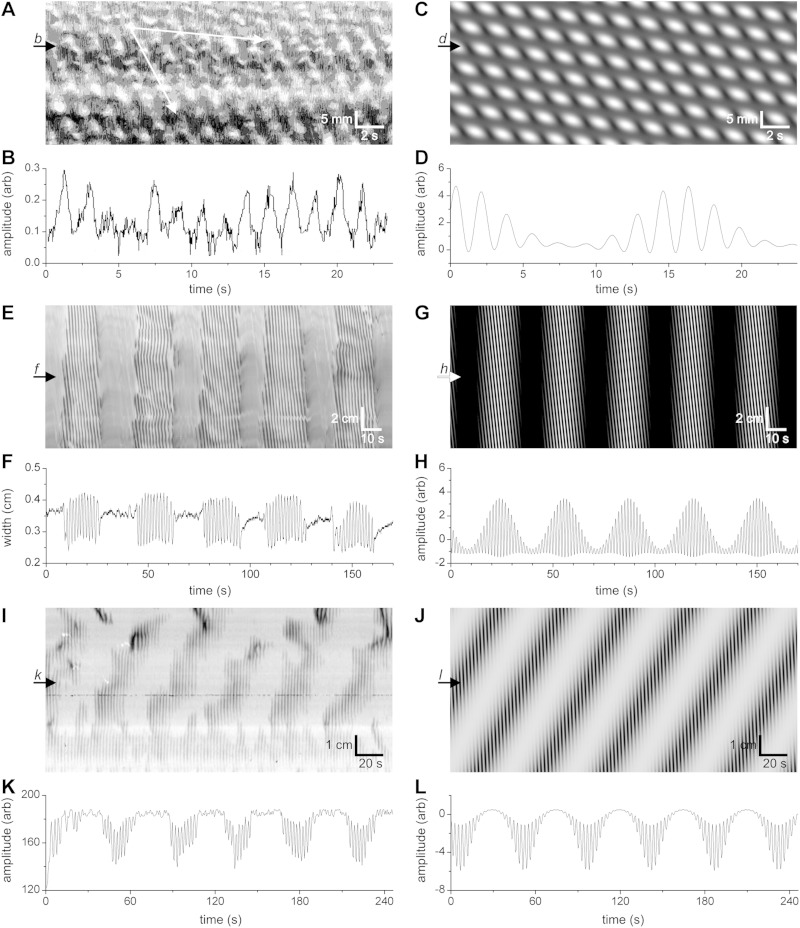
Spatiotemporal maps of mouse intestinal motor patterns and corresponding models. A and B: map and profile of the “Cannon-type” or “checkered” segmentation motor pattern of the small intestine. Top and bottom white arrows in A indicate presumed velocities of DMP and MP components, respectively. C and D: map and profile of model with parameters: Admp = 0.5; ωdmp = 4 min−1; vdmp = 0.04 cm/s; α = 1; ωmp = 34 min−1; vmp = 0.78 cm/s; B = 0; h = 5; c = 0.1; w = 0.18. E and F: map and profile of typical clustered propulsive activity of the small intestine. G and H: map and profile of model with parameters as follows: Admp = 2; ωdmp = 1.9 min−1; vdmp = 2 cm/s; α = 0; ωmp = 40 min−1; vmp = 1.65 cm/s; B = 0; h = 5; c = 0.5; w = 0.2 (see also Supplemental Movies S1 and S4). I and K: map and profile of clusters of short-lasting propagating slow-wave-driven contraction waves obtained from a typical experiment. The slow component (at the cluster frequency) propagates in retrograde direction. J and L: map and profile of model with parameters: Admp = 1; ωdmp = 1.33 min−1; vdmp = −0.13 cm/s; α = −1.2; ωmp = 23 min−1; vmp = 1.5 cm/s; B = 0; h = 5; c = 0.1; w = 0.15. (see also Supplemental Movie S3). Amplitude profiles at b, d, f, h, k, l are shown in B, D, F, H, K and L, respectively.
Changing the propagation velocity of the ICC-DMP-generated rhythmic transient depolarizations.
The propagation velocity of the slow-wave-driven propulsive activity in the intestine has been reported numerous times by many investigators and it is usually in the order of 1 cm/s (2, 6, 21, 32, 56). The propagation velocity of the second electrical pacemaker activity has never been measured directly and we discovered that the occurrence of the “Cannon-type” or “checkered” segmentation motor patterns was critically dependent on its propagation velocity. The segmentation motor pattern only happened when the propagation velocity was below 0.05 cm/s. With faster propagation velocities, between 0.08 and 0.5 cm/s, the motor pattern showed still segmentation, that is, no noninterrupted propagating activity was seen (Fig. 1M), but it did not have the “pure” checkered appearance (Fig. 1L). When the propagation velocity became close to the slow wave propagation velocity, the motor pattern showed the cluster-type propulsive activity (Fig. 1N; Supplemental Movie S4), the dominant propulsive activity of the small intestine, reported on numerous times in the literature (see discussion). Importantly, the prominent waxing and waning of the high-frequency amplitude (shown in Fig. 1H) did not change. This indicates that the two prominent motor patterns of the small intestine, the segmentation pattern and the clustered propulsive contractions, are both orchestrated by interactions of the two pacemakers, in concert with neural excitation.
Segmentation and propulsion orchestrated by pacemaker interaction, dependent on propagation velocity shown in spatiotemporal maps of segments of intestine and through modeling.
Figure 5A is a spatiotemporal map of contractile activity of the small intestine displaying the typical Cannon-type “checkered” segmentation motor pattern. The slow wave frequency of the mouse small intestine (32 cpm) can easily be recognized. The propagation velocity of the dominant slow wave in the intestine, and hence the propagation velocity of the slow-wave-driven circular muscle contractions is relatively constant at ∼1 cm/s (bottom white arrow in Fig. 5A). This checkered pattern was modeled with a sine wave of 34 cpm at 0.78 cm/s whose amplitude was modulated by the phase of a sine wave of 4 cpm and a velocity of 0.04 cm/s (Fig. 5C). The checkered pattern from the model (Fig. 5C) is strikingly similar to the checkered pattern in the spatiotemporal map of the segmentation motor pattern (Fig. 5A). The amplitude profile, when measured at a fixed point over time, showed the waxing and waning pattern (Fig. 5D), similar to the waxing and waning of the intestine diameter in the segmentation motor pattern (Fig. 5B). The slow propagation velocity can be recognized in the spatiotemporal maps (see top white arrow in Fig. 5A). The frequency of the low-frequency component can be recognized as the waxing and waning frequency when the amplitude profile is plotted (Fig. 5D).
When, in the model, the propagation velocity of the low-frequency component was sped up to 2 cm/s, the clustered propulsive activity developed (Fig. 5G). The amplitude profile of this activity showed waxing and waning (Fig. 3H). In order to document the clustered contractile activity of the small intestine, experiments were performed on 12 mouse intestines that showed the cluster frequency as 1.4 ± 0.2 cpm and the cluster propagation velocity was 1.1 ± 0.2 cm/s (Fig. 5, E and F). The frequency of the clusters in the rat intestine was 0.5 ± 0.2 cpm (n = 11). The amplitude profile shows a low-frequency component with superimposed high-frequency activity that displays waxing and waning. The motor pattern can be described as clusters of slow-wave-driven propagating activity. When these slow-wave-driven propulsive contractions merge, which happens when the amplitude becomes high, effectively a single contraction propagates at the cluster frequency.
Figure 5I shows a nonpropulsive motor pattern that can be described as segmentation although it is not a typical “Cannon-type” checkered pattern. The slow-wave-driven contractions are not as short lasting as in the checkered pattern; they are propagating over very short distances. An amplitude profile (Fig. 5K) readily shows low-frequency periodicity with a waxing and waning pattern. This motor pattern was modeled using a high frequency of 23 cpm at a velocity of -1.5 cm/s and a low-frequency component at 1.3 cpm with a velocity of −0.13 cm/s (retrograde) (Fig. 5J). The original motor pattern (Fig. 5I) was not uniform and hence it cannot be modeled perfectly with the current model; however the bands of segmental contractions can easily be recognized in the model spatiotemporal map (Fig. 5J), and the amplitude profile shows waxing and waning (Fig. 5L; Supplemental Movie S3).
DISCUSSION
Regular rhythmic waxing and waning of the amplitude of contractions of the small intestine can be recognized in figures of many publications on intestinal motility (5, 15, 30, 33, 49), but it is almost never commented upon, although the occurrence of waxing and waning of slow waves has been clearly demonstrated (14, 53). Here we demonstrate that the waxing and waning is a reflection of the origin of the motor pattern, namely the interaction of two pacemaker activities: it is the result of the modulation of the amplitude of the slow waves generated by ICC-MP by the phase of the rhythmic transient depolarizations proposed to be originating from the ICC-DMP. This interaction can result in the typical checkered segmentation motor pattern as described by Cannon (7, 25), but it can also result in a clustered propulsive motor pattern. Both motor patterns are associated with waxing and waning of the amplitude of the slow-wave-associated contractions. The only difference between the generation of these two functionally different motor patterns may be the propagation velocity of the ICC-DMP pacemaker activity, the transient rhythmic depolarizations.
The present study shows that the phase-amplitude coupling function can be derived from the original recordings. It shows that the depolarization phase or the rhythmic transient depolarizations causes a reduction in the amplitude of the slow wave. Of interest is the fact that the center of the phase-amplitude coupling function was shifted to the left, very similar to the function created by trial and error in the experiment shown in Fig. 1 to match the original recording. Hence the peak of the reduction in slow wave amplitude occurs at the depolarizing phase of the ICC-DMP activity (Fig. 1K). It will be important in future studies to assess the biological variability of the phase-amplitude coupling function and its consequences for motor patterns.
The relationship between the slow wave frequency and the frequency of the slow-wave-driven contractions.
Muscle contractions are generated by action potentials generated by the smooth muscle cells. Smooth muscle cells do not spontaneously generate action potentials; the resting membrane potential is too hyperpolarized for calcium channels to open. The smooth muscle cells wait for a stimulus that depolarizes them. This depolarization is usually provided by “slow waves” that propagate into the musculature after being initiated by ICC-MP. In the mouse proximal intestine, the frequency of the slow waves is ∼45 cpm; in the rat intestine it is ∼35 cpm (56). The frequency becomes progressively lower towards the distal intestine. Slow wave activity occurs continuously. However, slow-wave-provided depolarization may not be enough to cause action potential generation, and hence a second stimulus may be necessary to further depolarize the muscle cells; this is often provided by a neural stimulus, but it can also be another stimulus such as distension of the intestinal wall causing stretch of the muscle cells. Only if all slow waves pass the “mechanical threshold” is there a 1:1 relationship between electrical activity and contraction. The contraction frequency will not surpass the electrical frequency. If a second pacemaker creates a waxing and waning of the slow wave amplitude, then only some slow waves will surpass the mechanical threshold and those that do will do so to varying degrees. This means that the number of action potentials (or the force of contraction) that is generated by each slow wave will vary depending on for how long and to what extent the various slow waves are surpassing the threshold. This is why the mechanical activity has a waxing and waning appearance in amplitude when the electrical activity does.
Interaction between two electrical pacemaker activities penetrating the circular musculature.
The high-frequency slow wave activity originates from ICC-MP, propagates within the ICC-MP network, and from there the slow waves propagate into the circular musculature (42). The low-frequency component, the rhythmic transient depolarizations, comes from the ICC-DMP (25). ICC-DMP form a network (10) of long bipolar cells that are oriented circumferentially. The activity from the ICC-DMP presumably can propagate in oral or anal direction, and from the ICC-DMP network the activity will propagate into the circular muscle. Recording intracellularly from a muscle cell in the circular muscle layer of the small intestine, in between the ICC-DMP and ICC-MP, the signals from both sources will come together (25, 39). Here we provide additional evidence using modeling that the resulting waxing and waning is due to phase-amplitude coupling between the slow waves and the lower frequency rhythmic transient depolarizations. The waxing and waning pattern does not emerge by simple addition of the two electrical signals. In fact, we show here that the phase-amplitude relationship can be directly derived from the original electrical recordings. The “typical” segmentation motor pattern, seen as a “checkered” motor pattern and identical to the description of Cannon in 1902, can faithfully be reproduced using the model, but this pattern only occurs when the propagation velocity of the low frequency is <0.05 cm/s. This low velocity is easily recognized in the spatiotemporal maps of the segmentation motor pattern (Fig. 5A). When the propagation velocity is increased to a value similar to that of the slow wave activity, then the segmentation pattern is lost and the motor pattern shows a clustered pattern of propulsive activity (Fig. 5E); see Supplemental Movie S4.
The clustered pattern of activity is the major propulsive motor pattern of the intestine. Whole intestine motility in vitro revealed that this motor activity occurs at 0.5–2 cpm (15, 16, 24, 37, 40, 48). The frequency is variable and depends on spontaneous neural activity and level of distension. The frequency of the contractions within the clusters is that of the omnipresent slow wave, and their amplitude shows a waxing and waning pattern. Within a cluster, the slow-wave-associated contractions can be recognized associated with high-amplitude transient intraluminal pressures (Fig. 5E) (40).
Clustered contractions in the human small intestine.
The above-described clustered motor pattern is prominently present in the human intestine and appears particularly abundant postprandially. The clusters have a minute rhythm (0.5–1 cpm) (23, 27, 47). (17). Two contraction rhythms were identified, one around 12 cpm (11) (22), the other around 0.5–1 cpm (18, 50). The slow wave pacemaker activity in the human intestine occurs at 9–12 cpm (51) associated with ICC-MP (17). In one study, the cluster contractions were assumed to be stationary (23), in another deemed to propagate (27), suggesting that they may relate to segmentation as well as propulsion. It is of great interest to note that the present study shows that segmentation and clustered propulsion cannot be distinguished from a single manometry recording. Figure 1I illustrates a waxing and waning pattern recorded at a single site; this exact activity is recorded whether the motor pattern represents segmentation (Fig. 1L) or propulsion (Fig. 1N).
A recent paper by Kuizenga et al. (30) allows an interesting comparison between our data and data from a segment of the human small intestine in vitro using high-resolution manometry. In the human intestine, clustered contractions are prominent. The slow-wave-driven “ripples” can occur in clusters with a waxing and waning amplitude. The clusters propagate “at the lower end of 1–20 mm/s,” which is similar to the propagation of the ripple contractions at 4 mm/s, consistent with the hypothesis generated in the present study that the low and high frequency have a similar propagation velocity. In the Kuizenga paper, the clustered contractions are thought to be governed by a neural pacemaker since this type of contraction is abolished by lidocaine. This is a logical hypothesis and we have no evidence to dispute it, but an alternate explanation is possible. The clustered contractions can be governed by a neurally induced pacemaker in the ICC-DMP (25). Definitely, the clustered nature and the waxing and waning can be entirely myogenic since it can persist in the presence of lidocaine, particularly clear in figure 6C in Ref. 30. The clustered contractions can also occur more or less randomly (figure 3A in Ref. 30) strikingly resembling a segmentation patterns as shown in the present study.
What determines the propagation velocity?
The propagation velocity of a slow wave is often thought to be reflecting the speed of active propagation of the slow wave through the syncytium of the ICC network. Although the biophysical features that would allow for this, the ion channels, the intracellular calcium oscillations, and voltage- and ligand-driven ion channel opening and closing, are all actively involved in slow wave activity, the network as a whole is not a passive system waiting for a proximal pacemaker to propagate through the system. All ICC are active pacemaker cells that are synchronized as a system of coupled oscillators. This becomes apparent when the tissue is cut into pieces; the slow waves appear in all pieces without the need for any stimulus. Recent work from our laboratory provided more evidence that to understand the network properties of the ICC-MP one has to take into account that it is a system of coupled oscillators (26). Hence, the propagation velocity is apparent and due to phase coupling of a system of coupled oscillators with an intrinsic frequency gradient (12, 43). The propagation velocity was rather constant in our experiments and in many other studies in the literature at around 1 cm/s although in the cat it varies from 10 cm/s in the proximal end to 1 cm/s in the distal end (31). Features of coupled oscillators that influence the apparent propagation velocity are the intercellular coupling characteristics within the ICC network (properties of gap junctions and/or ephaptic coupling) (29), the resting membrane potential of the ICC (3), their refractory period characteristics (4) and frequency gradients (12).
The network characteristics of the ICC-DMP have not been studied. We hypothesize that the rhythmic transient depolarization propagate within this network and propagate from this network into the circular muscle cells. Whether the ICC-DMP pacemaker activity behaves as a system of coupled oscillators is not known. The rhythmic transient depolarizations are stimulus dependent, with the nervous system likely the major stimulant. Hence neural action on the ICC-DMP network may influence the characteristics of the network including coupling characteristics such as gap junction conductance, possibly similar to dopaminergic regulation of gap junction conductance in the retina (57). Interestingly, gap junctions connecting ICC-MP are rare in the mouse small intestine (45, 54), whereas the ICC-DMP are rich in gap junctions, far richer than the ICC-MP network. ICC-DMP are also abundantly innervated, much more dense compared with ICC-MP (44, 46). Hence the transition from propulsion to segmentation may involve neural modulation of gap junction conductance within the ICC-DMP network, thereby changing the propagation velocity of the rhythmic transient depolarizations.
GRANTS
This study was supported by Canadian Institutes of Health Research Grant MOP12874 to J. D. Huizinga, Natural Sciences and Engineering Research Council Grant 386877 to J. D. Huizinga, and National Natural Science Foundation of China Grant 81170249 to J.-H. Chen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.H., J.-H.C., and M.P. conception and design of research; J.D.H., J.-H.C., A.J.P., M.P., C.L., Y.Y., P.Y., Q.L., M.T., Y.F.Z., and D.W. performed experiments; J.D.H., S.P.P., A.J.P., M.P., C.L., Y.Y., P.Y., M.T., and Y.F.Z. analyzed data; J.D.H., S.P.P., J.-H.C., A.J.P., M.P., and C.L. interpreted results of experiments; J.D.H., S.P.P., A.J.P., C.L., Y.Y., and P.Y. prepared figures; J.D.H. and C.L. drafted manuscript; J.D.H., S.P.P., and J.-H.C. edited and revised manuscript; J.D.H., S.P.P., J.-H.C., A.J.P., M.P., C.L., Y.Y., P.Y., Q.L., M.T., Y.F.Z., and D.W. approved final version of manuscript.
Supplementary Material
Footnotes
Supplemental movies can be seen at the journal website (http://ajpcell.physiology.org). Four spatiotemporal maps are converted to movies. Upon opening the movies, the spatiotemporal map is shown together with the movie on top. While the movie is running a white line traverses the map, in synchrony with the movie. The movies show the changes in diameter along the intestinal segment over time. In the movies, black is contraction, white is relaxation. Note that the only difference between S1, S3 and S4 is in the vdmp, the apparent propagation velocity of the ICC-DMP component.
REFERENCES
- 1.Alvarez WC. The myogenic nature of the contractions. Am J Physiol 59: 421–430, 1922. [Google Scholar]
- 2.Angeli TR, O'Grady G, Du P, Paskaranandavadivel N, Pullan AJ, Bissett IP, Cheng LK. Circumferential and functional re-entry of in vivo slow-wave activity in the porcine small intestine. Neurogastroenterol Motil 25: e304–e314, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardakjian BL, El-Sharkawy TY, Diamant NE. Interaction of coupled nonlinear oscillators having different intrinsic resting levels. J Theor Biol 106: 9–23, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Bardakjian BL, Lau MY. The refractory properties of mapped clock oscillators representing smooth muscle electrical oscillations. Prog Clin Biol Res 327: 627–634, 1990. [PubMed] [Google Scholar]
- 5.Bian X, Ren J, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol 551: 309–322, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bortoff A. Propagation and electrical entrainment of intestinal slow waves. Am J Dig Dis 17: 311–316, 1972. [DOI] [PubMed] [Google Scholar]
- 7.Cannon WB. The movements of the intestines studied by means of the Roentgen rays. J Med Res 7: 72–75, 1902. [PMC free article] [PubMed] [Google Scholar]
- 8.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci 14: 506–515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers JD, Bornstein JC, Thomas EA. Multiple neural oscillators and muscle feedback are required for the intestinal fed state motor program. PLoS One 6: e19597, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen J. Gross and microscopic anatomy of the large intestine. In: The Large Intestine: Physiology, Pathophysiology, and Disease, edited by Phillips SF, Pemberton JH, Shorter RG. Raven, 1991. [Google Scholar]
- 11.Christensen J, Schedl HP, Clifton JA. The small intestinal basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with variety of diseases. Gastroenterology 50: 309–315, 1966. [PubMed] [Google Scholar]
- 12.Daniel EE, Bardakjian BL, Huizinga JD, Diamant NE. Relaxation oscillator and core conductor models are needed for understanding of GI electrical activities. Am J Physiol Gastrointest Liver Physiol 266: G339–G349, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Der-Silaphet T, Malysz J, Hagel S, Arsenault LA, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology 114: 724–736, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Diamant NE, Bortoff A. Nature of the intestinal slow-wave frequency gradient. Am J Physiol 216: 301–307, 1969. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlein HJ, Schemann M, Siegle ML. Motor patterns of small intestine determined by closely spaced extraluminal transducers and videofluoroscopy. Am J Physiol Gastrointest Liver Physiol 253: G259–G267, 1987. [DOI] [PubMed] [Google Scholar]
- 16.Ferens D, Baell J, Lessene G, Smith JE, Furness JB. Effects of modulators of Ca2+-activated, intermediate-conductance potassium channels on motility of the rat small intestine, in vivo. Neurogastroenterol Motil 19: 383–389, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gallego D, Malagelada C, Accarino A, De Giorgio R, Malagelada JR, Azpiroz F, Jimenez M. Nitrergic and purinergic mechanisms evoke inhibitory neuromuscular transmission in the human small intestine. Neurogastroenterol Motil 26: 419–429, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Gorard DA, Libby GW, Farthing MJ. Ambulatory small intestinal motility in “diarrhoea” predominant irritable bowel syndrome. Gut 35: 203–210, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwynne RM, Bornstein JC. Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292: G1162–G1172, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Gwynne RM, Thomas EA, Goh SM, Sjovall H, Bornstein JC. Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejunum. J Physiol 556: 557–569, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall KE, el-Sharkawy TY, Diamant NE. Vagal control of canine postprandial upper gastrointestinal motility. Am J Physiol Gastrointest Liver Physiol 250: G501–G510, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol 372: 501–520, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellstrom PM. Motility of small intestine: a case for pattern recognition. J Intern Med 237: 391–394, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Huizinga JD, Ambrous K, Der-Silaphet T. Co-operation between neural and myogenic mechanisms in the control of distension-induced peristalsis in the mouse small intestine. J Physiol 506: 843–856, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huizinga JD, Chen JH, Zhu YF, Pawelka A, McGinn RJ, Bardakjian BL, Parsons SP, Kunze WA, Wu RY, Bercik P, Khoshdel A, Chen S, Yin S, Zhang Q, Yu Y, Gao Q, Li K, Hu X, Zarate N, Collins P, Pistilli M, Ma J, Zhang R, Chen D. The origin of segmentation motor activity in the intestine. Nat Commun 5: 3326, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsons SP, Huizinga JD. Effects of gap junction inhibition on contraction waves in the murine small intestine in relation to coupled oscillator theory. Am J Physiol Gastrointest Liver Physiol 308: G287–G297, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husebye E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil 11: 141–161, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Husebye E, Engedal K. The patterns of motility are maintained in the human small intestine throughout the process of aging. Scand J Gastroenterol 27: 397–404, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Imtiaz MS, Zhao J, Hosaka K, von der Weid PY, Crowe M, and van Helden DF. Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J 92: 3843–3861, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuizenga MH, Sia TC, Dodds KN, Wiklendt L, Arkwright JW, Thomas A, Brookes SJ, Spencer NJ, Wattchow DA, Dinning PG, Costa M. Neurally mediated propagating discrete clustered contractions superimposed on myogenic ripples in ex vivo segments of human ileum. Am J Physiol Gastrointest Liver Physiol 308: G1–G11, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Lammers WJ, Stephen B. Origin and propagation of individual slow waves along the intact feline small intestine. Exp Physiol 93: 334–346, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Lammers WJ, Stephen B, Slack JR, Dhanasekaran S. Anisotropic propagation in the small intestine. Neurogastroenterol Motil 14: 357–364, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Madrid AM, Brahm J, Antezana C, Gonzalez-Koch A, Defilippi C, Pimentel C, Oksenberg D, Defilippi C. Small bowel motility in primary biliary cirrhosis. Am J Gastroenterol 93: 2436–2440, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Mane N, Gil V, Martinez-Cutillas M, Martin MT, Gallego D, Jimenez M. Dynamics of inhibitory co-transmission, membrane potential and pacemaker activity determine neuromyogenic function in the rat colon. Pflügers Arch 446: 2305–2321, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Mane N, Jimenez M. Interplay between myogenic pacemakers and enteric neurons determine distinct motor patterns in the rat colon. Neurogastroenterol Motil 26: 1508–1512, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Marple SL. Computing the discrete-time “analytic” signal via FFT. IEEE Trans Signal Process 47: 2600–2603, 1999. [Google Scholar]
- 37.Nieuwmeyer F, Ye J, Huizinga JD. Ava[L-Pro9,N-MeLeu10] substance P(7–11) (GR 73632) and Sar9, Met(O2)11 increase distention-induced peristalsis through activation of neurokinin-1 receptors on smooth muscle and interstitial cells of cajal. J Pharmacol Exp Ther 317: 439–445, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Pawelka A. Pacemaker Activity of ICC-DMP in the Small Intestine (Master's thesis) Hamilton, Canada: McMaster University, 2014. [Google Scholar]
- 39.Pawelka A, Huizinga JD. Induction of rhythmic transient depolarizations associated with waxing and waning of the slow wave activity in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 308: G427–G433, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Pistilli MJ. Mechanisms Underlying Rhythmic Activities in the Gastrointestinal Tract (Master's thesis). Hamilton, Canada: McMaster University, 2012. [Google Scholar]
- 41.Pluja L, Alberti E, Fernandez E, Mikkelsen HB, Thuneberg L, Jimenez M. Evidence supporting presence of two pacemakers in rat colon. Am J Physiol Gastrointest Liver Physiol 281: G255–G266, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Publicover NG. Generation and propagation of rhythmicity in gastrointestinal smooth muscle. In: Pacemaker Activity and Intercellular Communication, edited by Huizinga JD. Boca Raton, FL: CRC, 1995, p. 175–192. [Google Scholar]
- 43.Publicover NG, Sanders KM. Are relaxation oscillators an appropriate model of gastrointestinal electrical activity? Am J Physiol Gastrointest Liver Physiol 256: G265–G274, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Rumessen JJ, Mikkelsen HB, Thuneberg L. Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterology 102: 56–68, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Rumessen JJ, Thuneberg L. Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Scand J Gastroenterol Suppl 216: 82–94, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Rumessen JJ, Thuneberg L, Mikkelsen HB. Plexus muscularis profundus and associated interstitial cells. II. Ultrastructural studies of mouse small intestine. Anat Rec 203: 129–146, 1982. [DOI] [PubMed] [Google Scholar]
- 47.Samsom M, Smout AJ, Hebbard G, Fraser R, Omari T, Horowitz M, Dent J. A novel portable perfused manometric system for recording of small intestinal motility. Neurogastroenterol Motil 10: 139–148, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Sarna SK, Otterson MF. Small intestinal physiology and pathophysiology. Gastroenterol Clin North Am 18: 375–404, 1989. [PubMed] [Google Scholar]
- 49.Seerden TC, Lammers WJ, De Winter BY, De Man JG, Pelckmans PA. Spatiotemporal electrical and motility mapping of distension-induced propagating oscillations in the murine small intestine. Am J Physiol Gastrointest Liver Physiol 289: G1043–G1051, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Simren M, Castedal M, Svedlund J, Abrahamsson H, Bjornsson E. Abnormal propagation pattern of duodenal pressure waves in the irritable bowel syndrome (IBS) [correction of (IBD)]. Dig Dis Sci 45: 2151–2161, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Stathopoulos E, Schlageter V, Meyrat B, Ribaupierre Y, Kucera P. Magnetic pill tracking: a novel non-invasive tool for investigation of human digestive motility. Neurogastroenterol Motil 17: 148–154, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Summers RW, Anuras S, Green J. Jejunal manometry patterns in health, partial intestinal obstruction, and pseudoobstruction. Gastroenterology 85: 1290–1300, 1983. [PubMed] [Google Scholar]
- 53.Suzuki N, Prosser CL, DeVos W. Waxing and waning of slow waves in intestinal musculature. Am J Physiol Gastrointest Liver Physiol 250: G28–G34, 1986. [DOI] [PubMed] [Google Scholar]
- 54.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol 71: 1–130, 1982. [PubMed] [Google Scholar]
- 55.Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104: 1195–1210, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang XY, Lammers WJ, Bercik P, Huizinga JD. Lack of pyloric interstitial cells of Cajal explains distinct peristaltic motor patterns in stomach and small intestine. Am J Physiol Gastrointest Liver Physiol 289: G539–G549, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Xia XB, Mills SL. Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci 21: 791–805, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.