Abstract
Vi capsular polysaccharide is currently in use as a vaccine against human typhoid caused by Salmonella Typhi. The vaccine efficacy correlates with IgG anti-Vi Abs. We have recently reported that Vi can generate inflammatory responses through activation of the TLR2/TLR1 complex. In the present study, we show that immunization with Vi produces IgM as well as IgG Abs in wild type mice. This ability is not compromised in mice deficient in T cells. However, immunization of mice lacking the TLR adaptor protein, MyD88, with Vi elicits only IgM Abs. These results suggest that MyD88-dependent pro-inflammatory ability of the Vi vaccine might be vital in generating IgG Abs with this T-independent Ag.
Keywords: Salmonella Typhi, Vi polysaccharide vaccine, TLR, MyD88, anti-Vi antibodies
Introduction
Abs to polysaccharides are known to play a crucial role in controlling systemic infection with encapsulated bacteria.1 These Abs are generated independent of T-cell help that is normally provided to B cells during humoral response against protein Ags. Although the Ab response to these Ags is predominantly IgM, IgG Abs are also produced, albeit at low levels. Unlike T-dependent protein Ags, immunization with these T-independent polysaccharide Ags does not generate memory B cells.1 Many of these polysaccharide Ags carry repetitive determinants, which enables them to engage simultaneously multiple Ag receptors on B cells. This engagement constitutes the first signal for B-cell proliferation, and the second signal that is required for immunoglobulin secretion and class switching can be provided by TNF family members such as B lymphocyte stimulator/B-cell activating factor (BLyS/BAFF) and a proliferation-inducing ligand (APRIL), or by cytokines such as IL-5 and IFNs.2–4 Recent studies suggest that TLR activation might also contribute to Ab responses against polysaccharide Ags either directly through cross-talk with the B-cell receptor or through activation of dendritic cells and macrophages. The latter provide soluble mediators that can promote B cell differentiation, immunoglobulin secretion and isotype switching.5,6
Vi is a polymer of α-(1→4)-linked 2-acetamido-2-deoxy-D-galacturonic acid (GalpANAc) with variable O-acetylation at C-3. It forms a capsule around Salmonella Typhi, the causative agent of typhoid in humans.7 Vi protects S. Typhi from complement-mediated killing and from the action of anti-O (lipopolysaccharide (LPS)) Ab.8 Abs against this polysaccharide provide protection against typhoid and this protection correlates with IgG.9 Our recent study demonstrated that Vi can induce secretion of pro-inflammatory cytokines through engagement of the TLR2/TLR1 complex on monocytes.10 Schreibelt et al. have shown that Vi vaccine can induce expression of co-stimulatory molecules and secretion of cytokines from human monocyte-derived dendritic cells.11 In the present study, we show that pro-inflammatory ability of Vi might regulate switching of anti-Vi Abs to IgG type in mice immunized with this polysaccharide.
Materials and methods
Reagents
Vi capsular polysaccharide (Vi) derived from S. Typhi was obtained from Bharat Biotech International Limited (Hyderabad, India). It was dialyzed against PBS before use in cellular experiments. LPS isolated from S. Typhi was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Anti-mouse Ig Abs labeled with HRP were purchased from Jackson Laboratories (West Grove, PA, USA). Alkaline phosphatase (AP)-conjugated isotype-specific Abs were procured from Southern Biotech (Birmingham, AL, USA).
Mice
C57BL/6, MyD88 null, TCR β null and TCR δ null mice were obtained from Jackson laboratories (Bar Harbor, ME, USA) and maintained in the small animal facility of the National Institute of Immunology. Animal experiments were carried out according to the guidelines provided by the institutional animal ethics committee.
Analysis of anti-Vi Ab response in mice
Mice (5–8 per group) were immunized i.p. with 10 µg Vi dissolved in sterile PBS or with a mixture of Vi and LPS (10 µg each, both derived from S. Typhi). Sera samples were obtained from mice before immunization (d 0) and d 14 after immunization. IgM and IgG anti-polysaccharide Abs were determined by ELISA. Briefly, Vi was coated in a 96-well microtitre plate overnight at 4℃ (0.5 μg/50 µl/well diluted in 100 mM carbonate buffer, pH 9.5). The plate was washed twice with PBST (PBS containing 0.05% Tween-20) and blocked with PBS-BSA (PBS supplemented with 1% BSA; 100 μl/well) for 1 h at 37℃. Sera samples diluted in PBS-BSA were added to each well (50 µl/well) and the plate was incubated for 1 h at 37℃. Subsequently, the plate was washed and incubated with HRP or AP-labeled anti-mouse Ig Ab diluted in PBST-BSA-1% (PBS-BSA containing 0.05% Tween-20) for 1 h. The plate was washed thoroughly with PBST and the reaction was developed by adding freshly prepared substrate solution [75 µl/well; TMB/TBABH/H2O2 for HRP; TMB, 3,3’,5,5’-tetramethyl benzidine (41 mM); TBABH, tetrametylammonium borohydride (8.2 mM); and para-nitrophenyl phosphate for AP). The plates were read at 405 nm for AP and 450 nm for HRP. The absorbance values were plotted against sera dilutions and Ab titers were calculated from the linear portion of the curve, as described by Marshall et al.12 IgM and IgG anti-Vi mAbs previously generated in the laboratory were used for reference purposes.13
Western blotting
Different concentrations of LPS derived from S. Typhi (Sigma Chemical Co.) and Vi vaccine were separated in a 10% SDS-polyacrylamide gel, transferred to nitrocellulose and probed with anti-S. Typhi LPS mAb reported previously.14 The nitrocellulose was washed and incubated with HRP-labeled anti-mouse Ig Ab and after washing developed using enhanced chemiluminescence (ECL) reagent.
Stimulation of cells and determination of cytokines
Peritoneal cells obtained from WT and MyD88-deficient C57BL/6 mice were allowed to adhere to plastic for 1–2 h and stimulated with Vi (1 and 5 µg/ml) for 24 h. Cytokines, KC and IL-6, were determined in culture supernatants using commercially available ELISA kits from BD Biosciences (San Jose, CA, USA) following the manufacturer’s instructions.
Splenocytes (3 × 105 cells/well in a 96-well plate) isolated from C57BL/6 mice were stimulated with different concentrations of LPS and Vi for 48 h, after which cells were pulsed with 3H-thymidine. 20 h later, the amount of radioactivity incorporated in cells was determined by liquid scintillation spectroscopy (Perkin Elmer, Waltham, MA, USA). Data expressed as mean CPM ± SD of triplicate cultures are representative of three independent experiments.
Statistical analysis
Probability of significance was calculated by two tails with a type 3 test for unequal variances using Student’s t-test in Microsoft Excel (Redmond, WA, USA). P-Values < 0.05 were considered statistically significant.
Results and discussion
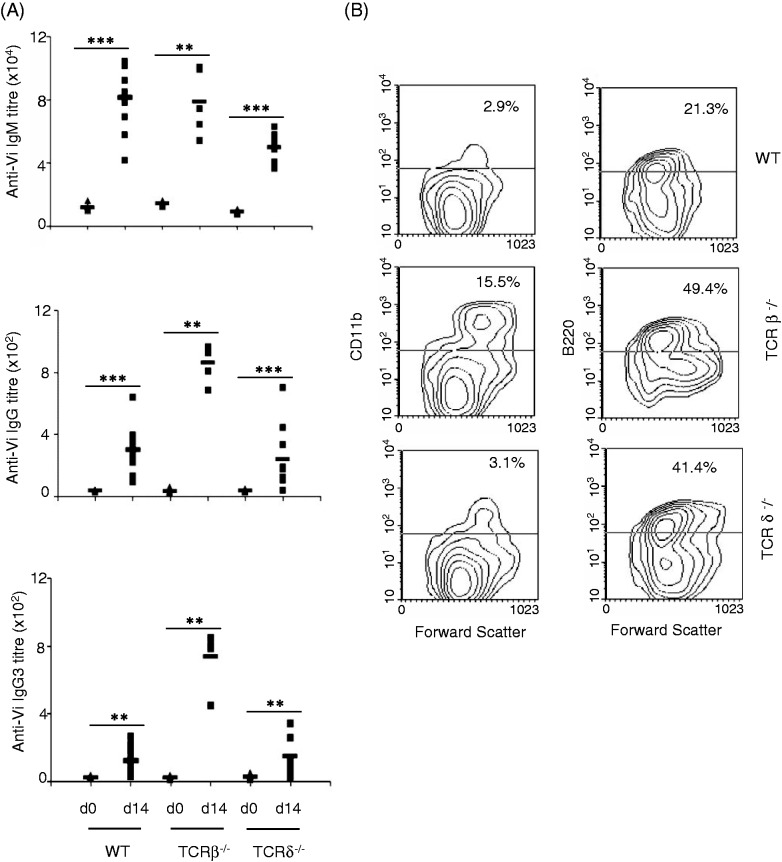
C57BL/6 mice immunized with Vi produced IgM, as well as IgG, Abs, with IgM levels being much higher (Figure 1A). Analysis of isotypes in sera obtained from these mice showed that IgG anti-Vi Abs were mainly of IgG3 type (Figure 1A). This response was not affected by the absence of T cells as mice lacking αβ T cells or γδ T cells also elicited similar Ab response upon immunization with Vi (Figure 1). In fact, IgG anti-Vi Abs were considerably higher in TCR β–/– as compared with wild type (WT) and TCR δ–/– mice (Figure 1A). These Abs were also mainly of IgG3 type (Figure 1A). In contrast, the levels of IgG anti-Vi Abs in TCR δ–/– mice were comparable with those seen in WT mice. These data suggested that T cells are not directly involved in bringing about switching of anti-Vi Abs to IgG, which is in agreement with a recent study by Marshall et al.12 We believe that increased IgG anti-Vi Ab levels in TCR β–/– mice might be due to increased number of inflammatory monocytes in these mice (Figure 1B). These increased numbers have been suggested to be an outcome of age-dependent deregulated inflammatory responses in these mice.15 These cells might have contributed to higher IgG anti-Vi Abs through amplified inflammatory responses with Vi.
Figure 1.
T cells do not contribute to Ab response against Vi. (A) C57BL/6 WT, TCR β–/– and TCR δ–/– mice were immunized intradermally with Vi (10 µg/mouse) and anti-Vi IgM and IgG Abs were determined in sera collected before immunization (d 0) and d 14 after immunization by ELISA. Sera were also analyzed for different IgG isotypes. (B) Splenocytes from WT, TCR β–/– and TCR δ–/– mice were stained with biotin-labeled anti-CD11b and anti-B220 Ab or isotype matched Ab followed by PE-conjugated avidin. Cells were washed and analyzed by flow cytometry. Data are representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
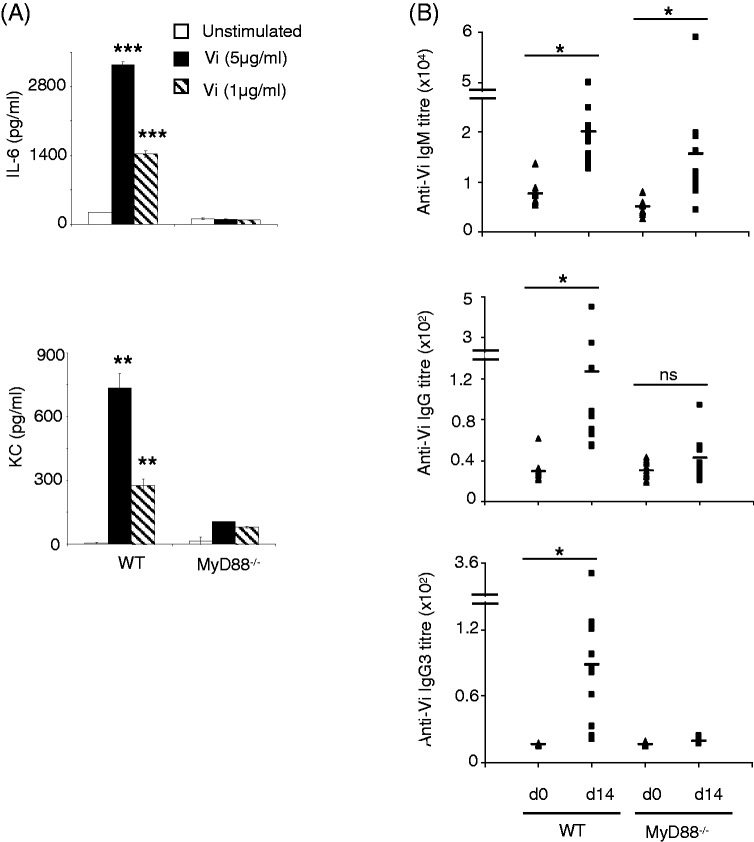
As Vi activates inflammatory responses from mononuclear phagocytes,10 we considered the possibility that these TLR-dependent responses might be involved in bringing about isotype switching during immunization with this vaccine. We therefore compared Ab response against Vi in WT mice and mice lacking the TLR adaptor, MyD88. Consistent with our previous findings,10 Vi induced secretion of KC and IL-6 from peritoneal cells derived from WT mice but not from MyD88 knockout mice (Figure 2A). Immunization of MyD88-deficient mice with Vi vaccine produced IgM anti-Vi Ab levels that were comparable with those seen in WT mice; however, unlike WT mice, MyD88-deficient mice showed negligible IgG anti-Vi Ab responses (Figure 2B), suggesting that MyD88-dependent TLR-stimulating activity might be crucial for generation of IgG anti-Vi Abs.
Figure 2.
MyD88-deficient mice do not produce IgG anti-Vi Abs. (A) Peritoneal cells were isolated from WT and MyD88-deficient C57BL/6 mice and stimulated in vitro with Vi for 24 h. KC and IL-6 levels were determined in cell culture supernatant by ELISA. Error bars represent SD. (B) WT and MyD88-deficient C57BL/6 mice were immunized with Vi (10 µg/mouse) i.p. and sera were analyzed for Vi-specific IgM and IgG Abs by ELISA. Data are representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. ns: not significant.
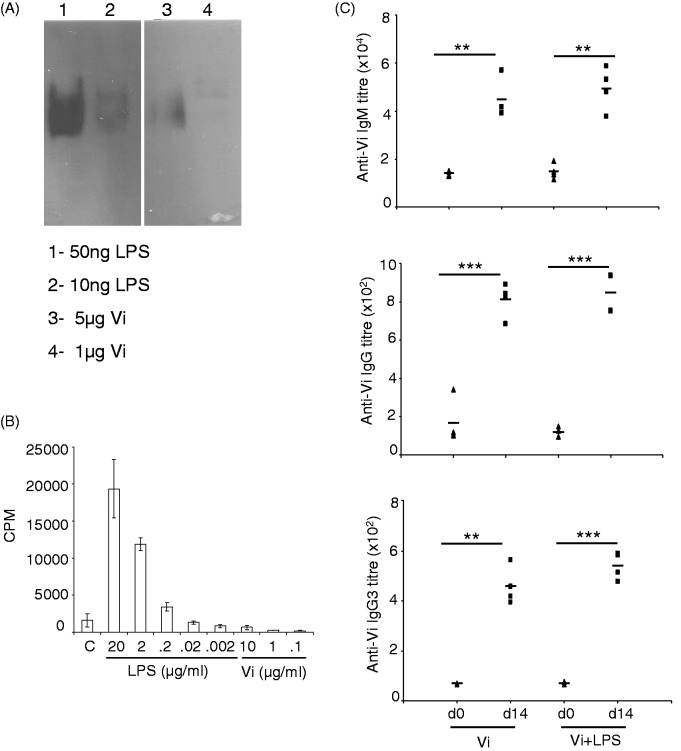
The Vi vaccine preparation used in this study contained about 0.2% LPS contamination (20 ng LPS in 10 µg Vi—the amount that was used in immunization experiments; Figure 3A). Such low levels of LPS are unlikely to have contributed to inflammatory responses that brought about isotype switching of anti-Vi Abs to IgG. LPS at < 200 ng/ml and Vi up to 10 µg/ml did not bring about proliferation of murine splenocytes in vitro, ruling out a possibility that LPS present in the Vi vaccine could stimulate proliferation of B cells and bring about isotype switching (Figure 3B). Further, immunization of mice with 10 µg Vi vaccine supplemented with additional 10 µg LPS isolated from S. Typhi did not lead to any measurable increase in anti-Vi IgG Ab response (Figure 3C). These data rule out involvement of LPS-activated responses in generation of anti-Vi IgG Abs in mice immunized with the Vi vaccine.
Figure 3.
LPS in the Vi vaccine preparation does not contribute to isotype switching of anti-Vi Abs. (A) LPS and Vi were separated in SDS-PAGE, transferred to nitrocellulose and probed with anti-LPS mAb. The blot was developed using ECL. (B) Splenocytes isolated from C57BL/6 mice were stimulated with different concentration of LPS and Vi. 48 h later, cells were pulsed with 3H-thymidine and the amount of radioactivity incorporated in cells was analyzed after 20 h by liquid scintillation spectroscopy. Data shown as mean cpm ± SD of triplicate cultures are representative of three independent experiments. (C) Mice were immunized with 10 µg Vi or 10 µg Vi supplemented with 10 µg LPS. Fourteen days later, Ab levels were determined in sera by ELISA. Data are representative of two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Abs to Vi protect humans against infection with S. Typhi and this protection is mainly conferred by IgG anti-Vi Abs.9,16 Szu et al. have shown that immunogenicity of Vi is closely related to the degree of O-acetylation,17 and we have shown that O-acetyl groups are required for the induction of TLR2-dependent inflammatory cytokines responses with Vi.10 The results presented here suggest that this inflammatory activity of Vi might regulate production of IgG Abs against this polysaccharide in mice. Whether this is through induction of pro-inflammatory cytokines from mononuclear phagocytes, which can bring about isotype switching with T-independent Ags, or through direct activation of TLR on Vi-specific B cells or through both these mechanisms needs to be investigated in future studies.
Inflammatory cytokines have been previously implicated in isotype switching with T-independent Ags in vitro and in vivo.5,6 Similarly, direct involvement of TLR agonists in promoting terminal plasma cell differentiation of B1 and marginal zone B cells, which are specialized in T-independent responses, has also been demonstrated.18 Quintana et al. have shown that anti-LPS specific IgG3 Abs might be generated as a result of collaboration between TLR4 and LPS-specific BCR.19 Therefore, it is possible that a similar kind of co-operation between TLR2 and BCR on specific B cells might bring about switching of anti-Vi Abs to IgG. The role of TLR in eliciting Ab responses with Vi has also been suggested by Majumder et al. through their analysis of single nucleotide polymorphism in humans.20 Taken together, our findings provide a previously unappreciated mechanism for generation of IgG Abs with Vi vaccine.
Acknowledgments
We would like to thank members of the Ayub laboratory for insightful discussions. RG and ASA received a research fellowship from Council of Scientific and Industrial Research, Govt. of India. The National Institute of Immunology is funded by the Department of Biotechnology, Government of India. The authors have no conflicting financial interests.
Footnotes
Rohini Garg is now at the National Institute of Plant and Genome Research, New Delhi, India. Rohini Garg and Ajay Suresh Akhade contributed equally to this work.
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Vos Q, Lees A, Wu ZQ, et al. B-cell activation by T-cell-independent type 2 Ags as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev 2000; 176: 154–170. [DOI] [PubMed] [Google Scholar]
- 2.Stein JV, Lopez-Fraga M, Elustondo FA, et al. APRIL modulates B and T cell immunity. J Clin Invest 2002; 109: 1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001; 293: 2111–2114. [DOI] [PubMed] [Google Scholar]
- 4.Snapper CM, McIntyre TM, Mandler R, et al. Induction of IgG3 secretion by interferon gamma: a model for T cell independent class switching in response to T cell-independent type 2 Ags. J Exp Med 1992; 175: 1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature 2005; 438: 364–368. [DOI] [PubMed] [Google Scholar]
- 6.Sen G, Khan AQ, Chen Q, et al. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol 2005; 175: 3084–3091. [DOI] [PubMed] [Google Scholar]
- 7.Felix A, Pitt RM. A new Ag of B. typhosus. Lancet 1934; 227: 186–191. [Google Scholar]
- 8.Looney RJ, Steigbigel RT. Role of the Vi Ag of Salmonella Typhi in resistance to host defence in vitro. J Lab Clin Med 1986; 108: 506–516. [PubMed] [Google Scholar]
- 9.Sur D, Ochiai RL, Bhattacharya SK, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med 2009; 361: 335–344. [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Qadri A. Hemoglobin transforms anti-inflammatory Salmonella Typhi virulence polysaccharide into a TLR-2 agonist. J Immunol 2010; 184: 5980–5987. [DOI] [PubMed] [Google Scholar]
- 11.Schreibelt G, Benitez-Ribas D, Schuurhuis D, et al. Commonly used prophylactic vaccines as an alternative for synthetically produced TLR ligands to mature monocytes-derived dendritic cells. Blood 2010; 116: 564–574. [DOI] [PubMed] [Google Scholar]
- 12.Marshall JL, Flores-Langarica A, Kingsley RA, et al. The capsular polysaccharide Vi from Salmonella Typhi is a B1b Ag. J Immunol 2012; 189: 5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qadri A, Ghosh S, Talwar GP. Monoclonal Abs against two discrete determinants on Vi capsular polysaccharide. J Immunoassay 1990; 11: 235–250. [DOI] [PubMed] [Google Scholar]
- 14.Qadri A, Gupta SK, Talwar GP. Monoclonal Abs delineate multiple epitopes on the O Ags of Salmonella Typhi lipopolysaccharide. J Clin Microbiol 1988; 26: 2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis 2003; 9: 246–259. [DOI] [PubMed] [Google Scholar]
- 16.Klugman KP, Gilbertson IT, Koornhof HJ, et al. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet 1987; 2: 1165–1169. [DOI] [PubMed] [Google Scholar]
- 17.Szu SC, Li XR, Stone AL, et al. Relation between structure and immunologic properties of Vi capsular polysaccharide. Infect Immun 1991; 59: 4555–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genestier L, Taillardet M, Mondiere P, et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol 2007; 178: 7779–7786. [DOI] [PubMed] [Google Scholar]
- 19.Quintana FJ, Solomon A, Cohen IR, et al. Induction of IgG3 to LPS via Toll-like receptor 4 co-stimulation. PloS One 2008; 3: e3509–e3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majumder PP, Staats HF, Sarkar-Roy N, et al. Genetic determinants of immune-response to a polysaccharide vaccine for typhoid. Hugo J 2009; 3: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]