Figure 5.
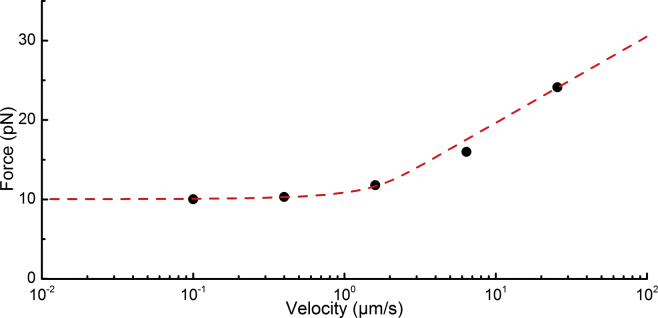
Dynamic force spectroscopy measurement of CS2 fimbria. The quaternary structure of an individual CS2 fimbria was unwound at velocities of 0.1, 0.4, 1.6, 6.4, and 25.6 μm/s for a distance of ∼2 μm and the corresponding force responses were sampled at 5 kHz. Each data point (black dots) shows the average unwinding force of fimbriae at five distinct velocities. For low velocities, i.e., velocities below the corner velocity, the unwinding force is independent of speed and amounts to 10 pN. The red dashed line shows a fit of the helix-like polymer model to the data, yielding the corner velocity, nm/s, and the bond length, nm, respectively. To see this figure in color, go online.
