FIG 2.
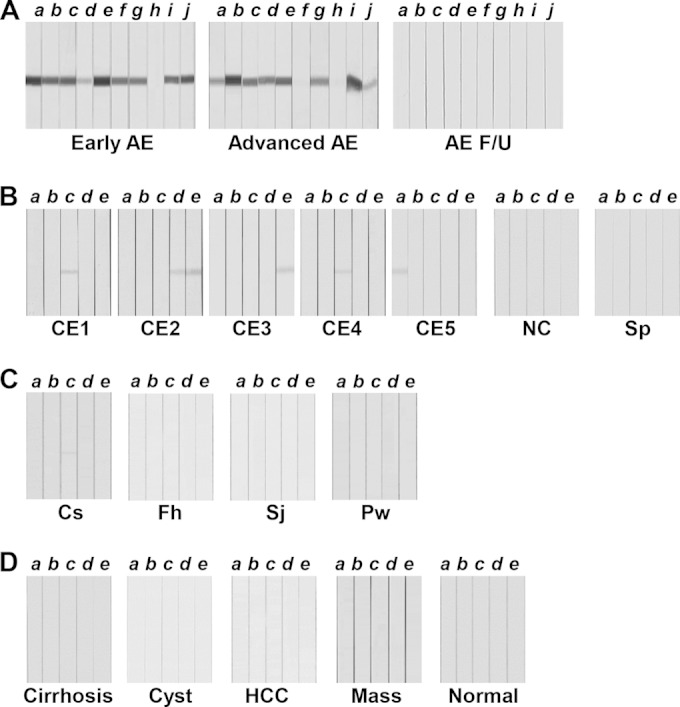
Assessment of the diagnostic reliability of recombinant EmAgB3 (rEmAgB3). rEmAgB3 protein separated by 12% SDS-PAGE was transferred to PVDF membranes. The membranes were cut into strips and incubated with individual patient sera. The strips were subsequently incubated with HRP-conjugated anti-human IgG and developed with 4C1N. (A) Immunoreactions of sera from patients with alveolar echinococcosis (AE). AE F/U, AE sera collected during follow-up monitoring. (B) Blots containing rEmAgB3 were probed with sera from patients with larval cestodiases, such as those seen in cases of cystic echinococcosis (CE; stages CE1 to CE5 are indicated), neurocysticercosis (NC), and sparganosis (Sp). (C) Sera from patients with clonorchiasis (Cs), fascioliasis (Fh), schistosomiasis japonicum (Sj), and paragonimiasis (Pw) were reacted with rEmAgB3. (D) Sera of patients with hepatic lesions, such as those with liver cirrhosis (Cirrhosis), simple liver cyst (Cyst), primary hepatocellular carcinoma (HCC), and liver mass (Mass), and sera from healthy controls (Normal) were incubated with rEmAgB3. Letters a to j indicate individual serum samples.
