Abstract
AIM: To evaluate the value of postprocessing techniques of CT colonography, including multiplanar reformation (MPR), virtual colonoscopy (VC), shaded surface display (SSD) and Raysum, in detection of colorectal carcinomas.
METHODS: Sixty-four patients with colorectal carcinoma underwent volume scanning with spiral CT. MPR, VC, SSD and Raysum images were obtained by using four kinds of postprocessing techniques in workstation. The results were comparatively analyzed according to circumferential extent, lesion length and pathology pattern of colorectal carcinomas. All diagnoses were proved pathologically and surgically.
RESULTS: The accuracy of circumferential extent of colorectal carcinoma determined by MPR, VC, SSD and Raysum was 100.0%, 82.8%, 79.7% and 79.7%, respectively. There was a significant statistical difference between MPR and VC. The consistent rate of lesion length was 89.1%, 76.6%, 95.3% and 100.0%, respectively. There was a statistical difference between VC and SSD. The accuracy of discriminating pathology pattern was 81.3%, 92.2%, 71.9% and 71.9%, respectively. There was a statistical difference between VC and SSD. MPR could determine accurately the circumference of colorectal carcinoma, Raysum could determine the length of lesion more precisely than SSD, VC was helpful in discriminating pathology patterns.
CONCLUSION: MPR, VC, SSD and Raysum have advantage and disadvantage in detection of colorectal carcinoma, use of these methods in combination can disclose the lesion more accurately.
INTRODUCTION
Multiplanar reformation (MPR), virtual colonoscopy (VC), shaded surface display (SSD) and Raysum images could be obtained after source data of CT colonography are processed in workstation. Numerous literatures on CT colonography are based on examination of colon polypi[1-17]. No research report on the diagnosis of colorectal carcinoma with postprocessing techiniques of MPR, VC, SSD and Raysum is available. The aim of this study was to investigate the clinical value of four postprocessing techniques in detection of colorectal carcinomas by comparing the results of 64 colorectal carcinomas.
MATERIALS AND METHODS
Clinical data
Sixty-four patients (39 men, 25 women, aged 20-78 years, mean age 55.6 years) with colorectal carcinomas were studied. All cases were diagnosed surgically and pathologically.
Examination protocol
The whole procedure of CT colonography included patient preparation, volume scanning and image postprocessing[18-20].
Patient preparation
A liquid diet for 48 h was used and 500 mL of 200 g/L mannite mixed with 1000 mL of 50 g/L glycol saline solution was administered orally in the evening prior to examination.
Anisodamine hydrochloride injection (654-2) (10 mg) was administered intramuscularly 10 min before CT scanning to alleviate colon spasm, minimize peristalsis and allow optimal colonic distention. The patient lay on right lateral decubitus position on CT table after dwelling a rectal enema tube. Then, the patient lay on supine, and room air was gently insufflated into the colorectum to distend the colon as long as the patient was tolerable.
Volume scanning
A HighSpeed advantage helical CT scanner (General Electric Medical System) was used to acquire a standard scout view image of the abdomen and pelvis to assess the degree of colorectal distension, room air was further insufflated if required. Images were acquired by using 3.0 mm collimation with a pitch of 2.0, 100-120 mA, 120 kV, and a 512 × 512 matrix. The range of scanning encompassed the entire colon from the rectum to cecum.
Image postprocessing
Image reconstruction data were transferred to a workstation (Sun Sparc 20 workstation, GE Advantage Windows 2.0 image analysis software) via picture archive and communication system, after retro-reconstructing the initial image data of scanning with 1.5 mm thickness, 0.5 mm interval. MPR, VC, SSD and Raysum images were obtained with postprocessing techniques in workstation.
MPR image: axial, coronal, sagittal and oblique images were acquired with the center on colorectal carcinoma segment by using CT software to display wall, lumen and adjacent structure of the lesion.
VC image: intraluminal image was obtained by applying Navigator software with about - 700 HU threshold from the rectum to cecum. Lesions were observed with Fly-through program along the longitudinal lumen[21].
SSD image: image reconstruction of colorectal area was performed with SSD software, then the interested colorectal segment were obtained by trimming off unnecessary part with Scalpel program, magnification and rotation were done to demonstrate colorectal carcinoma.
Raysum image: transparent image of interested colorectum was acquired by using Raysum software on the basis of SSD image to display the situation of endolumen and wall.
Statistical analysis
The following 3 aspects were comparatively analyzed according to surgery and pathology results. According to the invasive extent of colorectal carcinoma along the wall, tumors were divided into < 1/2, 1/2 - 3/4 and > 3/4 circumference. According to the longitudinal length of the lesion, it was classified into categories of 2.0-3.0 cm, 3.1-5.0 cm and 5.1-11.0 cm, respectively. The pathology pattern was classified into massive, ulcerous, infiltrative, ulcerous and infiltrative types.
Results of 4 kinds of postprocessing techniques were compared with surgical observation and pathology results. Accuracy of diagnosis with 4 kinds of postprocessing techniques was compared by using U test.
RESULTS
Examination results of circumferential extents, lesion lengths and pathology patterns in 64 colorectal carcinomas with 4 kinds of postprocessing techniques (Table 1)
Table 1.
Circumferential extents, lesion lengths and pathology patterns in 64 colorectal carcinomas determined with postprocessing techniques
| Item |
Diagnostic accordance (n) |
Accurate diagnosis (%) |
|||||||
| S&p c | MPR | VC | SSD | Raysum | MPR | VC | SSD | Raysum | |
| Circumf extent | 64 | 64 | 53 | 51 | 51 | 100.0 | 82.8 | 79.7 | 79.7 |
| < 1/2 circum | 7 | 7 | 6 | 6 | 6 | 100.0 | 85.7 | 85.7 | 85.7 |
| 1/2-3/4 circum | 14 | 14 | 12 | 11 | 11 | 100.0 | 85.7 | 78.6 | 78.6 |
| > 3/4 circum | 43 | 43 | 35 | 34 | 34 | 100.0 | 81.4 | 79.1 | 79.1 |
| Lesion length (cm) | 64 | 57 | 49 | 61 | 64 | 89.1 | 76.6 | 95.3 | 100.0 |
| 2.0-3.0 | 6 | 6 | 6 | 6 | 6 | 100.0 | 100.0 | 100.0 | 100.0 |
| 3.1-5.0 | 33 | 31 | 26 | 32 | 33 | 93.9 | 78.8 | 97.0 | 100.0 |
| 5.1-11.0 | 25 | 20 | 17 | 23 | 25 | 80.0 | 68.0 | 92.0 | 100.0 |
| Pathology pattern | 64 | 52 | 59 | 46 | 46 | 81.2 | 92.2 | 71.9 | 71.9 |
| Massive type | 38 | 32 | 38 | 27 | 27 | 84.2 | 100.0 | 71.1 | 71.1 |
| Ulcerous type | 5 | 4 | 4 | 3 | 4 | 80.0 | 80.0 | 60.0 | 80.0 |
| Infiltrative type | 13 | 11 | 12 | 11 | 12 | 84.6 | 92.3 | 84.6 | 92.3 |
| Ulc and inf type | 8 | 5 | 5 | 5 | 3 | 62.5 | 62.5 | 62.5 | 37.5 |
S & p c = Surgery and pathology case, Circumf = Circumferential, circum = circumference, Ulc and inf = Ulcerous and infiltrative. For circum-ferential extent, comparison between MPR and VC, U = 3.472, P < 0.001; comparison between VC and SSD, U = 0.449, P > 0.05; comparison between SSD and Raysum, U = 0.000, P > 0.05. For Lesion length, comparison between MPR and VC, U = 1.875, P > 0.05; comparison between VC and SSD, U = 3.034, P < 0.05; comparison between SSD and Raysum U = 1.759, P > 0.05. For pathologic pattern, compari-son between MPR and VC, U = 1.817, P > 0.05; comparison between VC and SSD, U = 2.991, P < 0.05; comparison between SSD and Raysum, U = 0.000, P > 0.05.
Diagnostic accordance of 64 colorectal carcinomas with 4 kinds of postprocessing techniques (Table 2)
Table 2.
Diagnosis accordance of 64 colorectal carcinomas with postprocessing techniques
| Postprocessing technique |
Circumferential extent |
Lesion length |
Pathological pattern |
|||
| Case (n) | % | Case (n) | % | Case (n) | % | |
| MPR | 64 | 100.0 | 57 | 89.1 | 52 | 81.2 |
| VC | 53 | 82.8 | 49 | 76.6 | 59 | 92.2 |
| SSD | 51 | 79.7 | 61 | 95.3 | 46 | 71.9 |
| Raysum | 51 | 79.7 | 64 | 100.0 | 46 | 71.9 |
| χ2 value | 14.748b | 22.430b | 10.909a | |||
P < 0.05 vs pathological pattern,
P < 0.01 vs circumferential extent and lesion length.
DISCUSSION
MPR, VC, SSD and Raysum images obtained by postprocessing technique displayed colorectal carcinoma in different manners with different clinical values.
Circumferential extent
The accuracy of circumferential extent of colorectal carcinoma determined with MPR, VC, SSD and Raysum was 100.0%, 82.8%, 79.7% and 79.7%, respectively (Tables 1, Tables 2). Significant statistical difference between MPR and VC, MPR and SSD, MPR and Raysum were obtained, respectively.
MPR had two dimensional axial, coronal, sagittal and oblique reconstruction images in series, on the center of colorectal carcinoma segments. MPR might reflect different density tissues by using different attenuation scales with high density resolution and it has no obvious artifact. It could clearly display intraluminal lesion and range invaded by carcinoma along its wall and adjacent structure, and accurately determine the circumferential extent of colorectal carcinoma[22].
VC, SSD and Raysum images could be obtained by using appropriate CT threshold values with transparence of the part beyond them, could make use of only certain information without favorable disclosure of lesions in detail. Sometimes, they had difficulty in showing directly the condition of colorectal wall when thickening was not obvious. Therefore, the determination of circumferential extent of colorectal carcinoma was not so accurate as MPR (Figure 1A-C).
Figure 1.
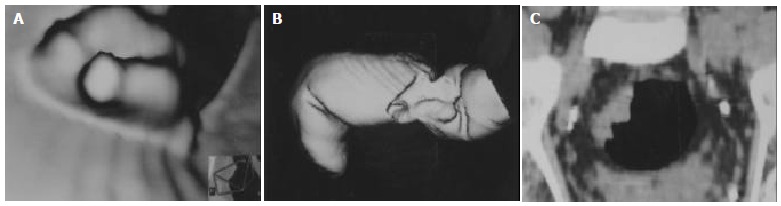
Massive rectal carcinoma in a 74 years old man. VC, SSD displayed an irregular mass, but unable to determine circumfer-ential extent (A, B). MPR showed the mass with 1/4 circumference around rectal wall (C).
Length of tumors
The correction rate of lesion lengths determined with MPR, VC, SSD and Raysum was 89.1%, 76.6%, 95.3% and 100.0%, respectively (Tables 1, Tables 2).
SSD image displayed the surface of colorectal lumen from outside to inside, being similar to the image of filling phase in double contrast barium enema. It could be locally magnified and rotated polygonally to demonstrate colorectal carcinoma as clear as possible and show lesion lengths and morphology of two ends. But it utilized only certain information and did not reveal the lesion in detail due to the appropriate CT threshold value and transparence of the part beyond it. Moreover, because of partial covering of lesions by the colorectum, sometimes its manifestation was not quite precise[23]. Its correction rate of lesion lengths was 95.3 % in our series.
Raysum was obtained on the basis of SSD image similar to the image of mucosa phase in double contrast barium enema. It could display the situation of endolumen and wall by transparence and avoid the disadvantages of partial overlapping of lesions by colorectum with SSD, hence clearly revealing lesion lengths and morphology at two ends and accurately manifesting the lesion lengths[24] (Figure 2A, B).
Figure 2.
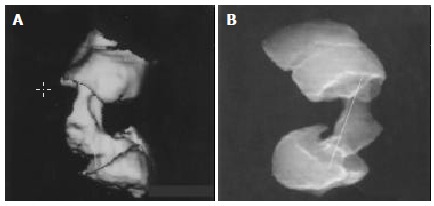
Infiltrative rectal carcinoma in a 46 years old man. SSD disclosed the two ends of carcinoma and measured its length (A). Raysum manifested the two ends of carcinoma more clearly and measured its length more accurately than SSD (B).
MPR was a two dimensional image formed by reconstruction. But the colorectum was tortuous in structure, moreover, it was complicated by the presence of colorectal carcinoma. Therefore, measurement of the lesion length with MPR was not accurate[25]. In our study its accuracy was 89.1%.
VC could obtain virtual cubic images from colorectal endolumen. Its image was similar to that of fiberoptic colonoscopy. It could directly show the surface morphology of colorectal carcinoma, and its distal and proximal situations. But its manifestation of lesion lengths had a comparatively great error[3,4,14,18,26-28]. The correct rate was only 76.6% in our series.
Pathological patterns
The accuracy of pathology patterns determined by MPR, VC, SSD and Raysum was 81.3%, 92.2%, 71.9% and 71.9%, respectively (Tables 1, Tables 2). Significant statistical differences between VC and SSD were noticed.
VC image could disclose the surface morphology of colorectal carcinoma and its distal and proximal situations directly with observations from endolumen, favoring the discrimination of pathology patterns. However, there was certain difficulty in revealing the detail of carcinoma. It had errors in discriminating pathology patterns[29-30]. The correction rate was 92.2% in our study.
MPR was a two-dimensional image formed by reconstruction, and was combined with axial, coronal, sagittal and oblique images. But it had no direct three dimensional manifestation with a relative great error in discriminating pathology patterns (Figure 3A-C).
Figure 3.
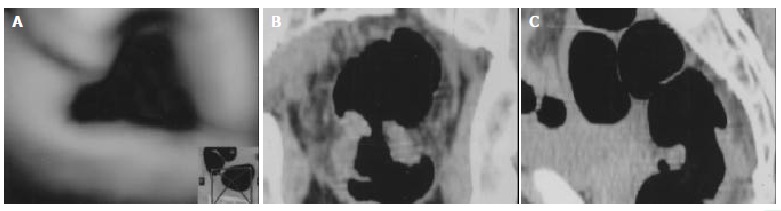
Infiltrative rectal carcinoma in a 48 years old man. VC demonstrated the carcinoma around rectal wall (A). Combination of coronal and sagittal images of MPR revealed an infiltrative carcinoma, but was not so obvious as VC (B, C).
SSD, Raysum images could display the surface of colorectal lumen from outside to inside, similar to the images of filling and mucosa phase in double contrast barium enema. But they could use certain information and could not show carcinoma details, and were fairly inaccurate in discriminating pathology patterns. Its correction rate was only 71.9% in our series.
In conclusion, MPR, VC, SSD and Raysum have both advantage and disadvantage in detection of colorectal carcinoma. MPR can accurately determine the circumferential extent, Raysum can fairly determine the length of lesions, and is more trustworthy than SSD, VC is helpful in discriminating pathology patterns. Their combination can disclose colorectal carcinomas more accurately.
ACKNOWLEDGEMENTS
The authors are grateful to Zhongshan Hospital, Fudan University Medical School, Shanghai 200032, China, for its help in accomplishing of the study.
Footnotes
Edited by Wang XL Proofread by Xu FM
References
- 1.Podolsky DK. Going the distance--the case for true colorectal-cancer screening. N Engl J Med. 2000;343:207–208. doi: 10.1056/NEJM200007203430309. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher RH. The end of barium enemas. N Engl J Med. 2000;342:1823–1824. doi: 10.1056/NEJM200006153422409. [DOI] [PubMed] [Google Scholar]
- 3.Bond JH. Virtual colonoscopy--promising, but not ready for widespread use. N Engl J Med. 1999;341:1540–1542. doi: 10.1056/NEJM199911113412010. [DOI] [PubMed] [Google Scholar]
- 4.Fenlon HM, Nunes DP, Schroy PC, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med. 1999;341:1496–1503. doi: 10.1056/NEJM199911113412003. [DOI] [PubMed] [Google Scholar]
- 5.Zalis ME, Perumpillichira J, Del Frate C, Hahn PF. CT colonography: digital subtraction bowel cleansing with mucosal reconstruction initial observations. Radiology. 2003;226:911–917. doi: 10.1148/radiol.2263012059. [DOI] [PubMed] [Google Scholar]
- 6.Yee J, Kumar NN, Hung RK, Akerkar GA, Kumar PR, Wall SD. Comparison of supine and prone scanning separately and in combination at CT colonography. Radiology. 2003;226:653–661. doi: 10.1148/radiol.2263010701. [DOI] [PubMed] [Google Scholar]
- 7.Summers RM, Jerebko AK, Franaszek M, Malley JD, Johnson CD. Colonic polyps: complementary role of computer-aided detection in CT colonography. Radiology. 2002;225:391–399. doi: 10.1148/radiol.2252011619. [DOI] [PubMed] [Google Scholar]
- 8.McFarland EG, Pilgram TK, Brink JA, McDermott RA, Santillan CV, Brady PW, Heiken JP, Balfe DM, Weinstock LB, Thyssen EP, et al. CT colonography: multiobserver diagnostic performance. Radiology. 2002;225:380–390. doi: 10.1148/radiol.2252011625. [DOI] [PubMed] [Google Scholar]
- 9.Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, Van Holsbeeck BG. Dietary fecal tagging as a cleansing method before CT colonography: initial results polyp detection and patient acceptance. Radiology. 2002;224:393–403. doi: 10.1148/radiol.2241011222. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida H, Masutani Y, MacEneaney P, Rubin DT, Dachman AH. Computerized detection of colonic polyps at CT colonography on the basis of volumetric features: pilot study. Radiology. 2002;222:327–336. doi: 10.1148/radiol.2222010506. [DOI] [PubMed] [Google Scholar]
- 11.Yee J, Akerkar GA, Hung RK, Steinauer-Gebauer AM, Wall SD, McQuaid KR. Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Radiology. 2001;219:685–692. doi: 10.1148/radiology.219.3.r01jn40685. [DOI] [PubMed] [Google Scholar]
- 12.Summers RM, Johnson CD, Pusanik LM, Malley JD, Youssef AM, Reed JE. Automated polyp detection at CT colonography: feasibility assessment in a human population. Radiology. 2001;219:51–59. doi: 10.1148/radiology.219.1.r01ap0751. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci JT. Colon cancer screening with virtual colonoscopy: promise, polyps, politics. AJR Am J Roentgenol. 2001;177:975–988. doi: 10.2214/ajr.177.5.1770975. [DOI] [PubMed] [Google Scholar]
- 14.Luo MY, Zhou KR, Yao LQ. Experimental study of simulated polyp in pig colon: detection with CT virtual colonoscopy. Zhongguo Yixue Yingxiang Jishu. 2000;16:719–721. [Google Scholar]
- 15.Luo MY, Zhou KR, Yao LQ. Comparative study on the detecting colorectal polyps with virtual colonoscopy or other postprocessing techniques. Linchuang Fangshexue Zazhi. 2000;19:699–702. [Google Scholar]
- 16.Summers RM, Beaulieu CF, Pusanik LM, Malley JD, Jeffrey RB, Glazer DI, Napel S. Automated polyp detector for CT colonography: feasibility study. Radiology. 2000;216:284–290. doi: 10.1148/radiology.216.1.r00jl43284. [DOI] [PubMed] [Google Scholar]
- 17.Macari M, Milano A, Lavelle M, Berman P, Megibow AJ. Comparison of time-efficient CT colonography with two- and three-dimensional colonic evaluation for detecting colorectal polyps. AJR Am J Roentgenol. 2000;174:1543–1549. doi: 10.2214/ajr.174.6.1741543. [DOI] [PubMed] [Google Scholar]
- 18.Luo M, Shan H, Zhou K. CT virtual colonoscopy in patients with incomplete conventional colonoscopy. Chin Med J (Engl) 2002;115:1023–1026. [PubMed] [Google Scholar]
- 19.Zalis ME, Hahn PF. Digital subtraction bowel cleansing in CT colonography. AJR Am J Roentgenol. 2001;176:646–648. doi: 10.2214/ajr.176.3.1760646. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher JG, Johnson CD, Welch TJ, MacCarty RL, Ahlquist DA, Reed JE, Harmsen WS, Wilson LA. Optimization of CT colonography technique: prospective trial in 180 patients. Radiology. 2000;216:704–711. doi: 10.1148/radiology.216.3.r00au41704. [DOI] [PubMed] [Google Scholar]
- 21.Neri E, Giusti P, Battolla L, Vagli P, Boraschi P, Lencioni R, Caramella D, Bartolozzi C. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology. 2002;223:615–619. doi: 10.1148/radiol.2233010928. [DOI] [PubMed] [Google Scholar]
- 22.McFarland EG, Brink JA, Pilgram TK, Heiken JP, Balfe DM, Hirselj DA, Weinstock L, Littenberg B. Spiral CT colonography: reader agreement and diagnostic performance with two- and three-dimensional image-display techniques. Radiology. 2001;218:375–383. doi: 10.1148/radiology.218.2.r01ja47375. [DOI] [PubMed] [Google Scholar]
- 23.Hopper KD, Iyriboz AT, Wise SW, Neuman JD, Mauger DT, Kasales CJ. Mucosal detail at CT virtual reality: surface versus volume rendering. Radiology. 2000;214:517–522. doi: 10.1148/radiology.214.2.r00fe34517. [DOI] [PubMed] [Google Scholar]
- 24.Glick S. Double-contrast barium enema for colorectal cancer screening: a review of the issues and a comparison with other screening alternatives. AJR Am J Roentgenol. 2000;174:1529–1537. doi: 10.2214/ajr.174.6.1741529. [DOI] [PubMed] [Google Scholar]
- 25.Macari M, Megibow AJ. Pitfalls of using three-dimensional CT colonography with two-dimensional imaging correlation. AJR Am J Roentgenol. 2001;176:137–143. doi: 10.2214/ajr.176.1.1760137. [DOI] [PubMed] [Google Scholar]
- 26.Makin GB, Breen DJ, Monson JR. The impact of new technology on surgery for colorectal cancer. World J Gastroenterol. 2001;7:612–621. doi: 10.3748/wjg.v7.i5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spinzi G, Belloni G, Martegani A, Sangiovanni A, Del Favero C, Minoli G. Computed tomographic colonography and conventional colonoscopy for colon diseases: a prospective, blinded study. Am J Gastroenterol. 2001;96:394–400. doi: 10.1111/j.1572-0241.2001.03550.x. [DOI] [PubMed] [Google Scholar]
- 28.Mendelson RM, Foster NM, Edwards JT, Wood CJ, Rosenberg MS, Forbes GM. Virtual colonoscopy compared with conventional colonoscopy: a developing technology. Med J Aust. 2000;173:472–475. doi: 10.5694/j.1326-5377.2000.tb139298.x. [DOI] [PubMed] [Google Scholar]
- 29.Pescatore P, Glücker T, Delarive J, Meuli R, Pantoflickova D, Duvoisin B, Schnyder P, Blum AL, Dorta G. Diagnostic accuracy and interobserver agreement of CT colonography (virtual colonoscopy) Gut. 2000;47:126–130. doi: 10.1136/gut.47.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson CD, Ahlquist DA. Computed tomography colonography (virtual colonoscopy) : a new method for colorectal screening. Gut. 1999;44:301–305. doi: 10.1136/gut.44.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


