Abstract
A juvenile Australian shepherd dog exhibited failure to grow, inappetence, weakness, nonregenerative anemia, neutropenia, and cobalamin deficiency. DNA testing confirmed homozygosity of an amnionless mutation (AMN c.3G > A). Clinical signs resolved with supportive care and parenteral cobalamin supplementation. Inherited selective intestinal cobalamin malabsorption requiring lifelong parenteral supplementation should be considered in Australian shepherds, giant schnauzers, border collies, and beagles that fail to thrive.
Résumé
Gestion efficace d’un retard de croissance et de complications potentiellement mortelles attribuables à une malabsorption de cobalamine sélective héréditaire chez un jeune chien Berger australien. Un jeune chien Berger australien a manifesté une absence de croissance, de l’inappétence, de la faiblesse, une anémie non régénérative, de la neutropénie et une déficience de cobalamine. Des tests d’ADN ont confirmé l’homozygotisme d’une mutation des récepteurs amnionless (AMN c.3G > A). Les signes cliniques se sont résorbés avec des soins de soutien et des suppléments de cobalamine parentéraux. Une malabsorption intestinale sélective héréditaire de cobalamine exigeant des suppléments parentéraux à vie devrait être considérée chez les Bergers australiens, les Schnauzers géants, les Border-Collies et les Beagles qui manifestent des problèmes de croissance.
(Traduit par Isabelle Vallières)
Cobalamin (vitamin B12) is an essential micronutrient required for maintenance of normal physiologic functions including nucleic acid synthesis, amino acid metabolism, intestinal epithelial function, and hematopoiesis (1). The vitamin is synthesized in nature only by certain bacteria (e.g., Propionibacterium shermanii, Clostridium spp.) and must be absorbed from the diet by monogastric animals. Selective intestinal cobalamin malabsorption is an autosomal recessive disorder in Australian shepherds, giant schnauzers, border collies, and beagles (2–8). The canine disorder is an ortholog of Imerslund-Gräsbeck syndrome (I-GS) in humans, in which congenital intestinal malabsorption of cobalamin leads to undetectable serum cobalamin concentrations, methylmalonic acidemia/aciduria, homocysteinemia, dyshematopoiesis, and developmental delay (9,10). Juvenile Australian shepherds affected by this condition display similar signs of cobalamin deficiency, and the molecular basis of their disorder was identified as a mutation of the amnionless gene (AMN c.3G > A) (2). Unrecognized and untreated cobalamin deficiency may lead to lethal metabolic decompensation.
Fortunately, timely parenteral cobalamin supplementation can reverse severe metabolic and hematologic derangements, and lifelong treatment ensures normal life expectancy. Therefore, prompt recognition of the disease and parenteral cobalamin supplementation are essential to ensure that this easily treatable but vague clinical syndrome is not fatal.
Case description
A young female/intact Australian shepherd dog was referred to the Michigan State University Veterinary Medical Center (MSU-VMC) for evaluation of failure to grow, inappetence, lethargy, anemia, and leukopenia. Other findings from the referring veterinarian included lack of parasite ova in feces and mild elevations of serum bile acids and ACTH-stimulated serum cortisol. Since the time of adoption at 8 wk of age the dog had vacillated between periods of lethargy and normal activity. On presentation to the MSU-VMC at 5 mo of age the dog weighed 3.6 kg, was inactive, had poor body condition (BCS 3/9), and was estimated to be 7% dehydrated based on dry mucous membranes and moderate loss of skin turgor. Vital parameters, thoracic auscultation, abdominal palpation, and neurologic examination were within normal limits. When encouraged to walk, the dog had a hunched back and took short strides.
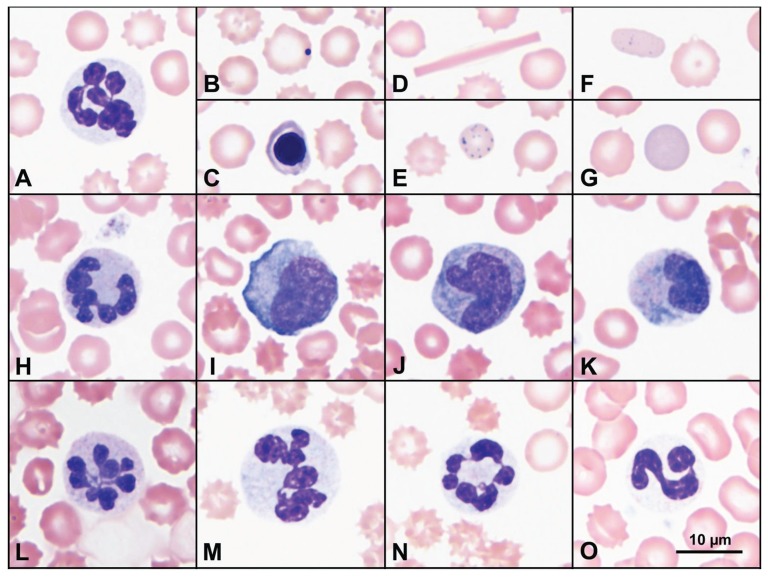
A complete blood (cell) count (CBC) on day 1 revealed a normocytic hypochromic nonregenerative anemia (see Table 1 for laboratory values) and leukopenia characterized by marked neutropenia and moderate lymphopenia. Blood smear examination revealed several hypersegmented neutrophils with mild, diffuse cytoplasmic basophilia (Figure 1). There was a mild rubricytosis (0.2 × 103/μL), an increase in Howell-Jolly bodies, rare basophilic stippling, and rare polychromatophilic erythrocytes that tended to be similar in size to mature erythrocytes. Mild keratocytosis and fragmentation may have been masked to some degree by marked echinocytosis, and widely scattered erythrocytes contained short to long rectangular hemoglobin crystals. Band neutrophils were not detected.
Table 1.
Abnormal laboratory values observed during diagnosis and treatment of a young Australian shepherd dog with inherited cobalamin malabsorption
| Reference | Day 1* | Day 3# | Day 5 | Day 7 | Day 15 | |
|---|---|---|---|---|---|---|
| Hematology | ||||||
| Hematocrit (%) | 40–55 | 19 | 27 | 25 | 21 | 31 |
| Reticulocytes (×104/μL) | 1.20–7.60 | 1.59 | < 1.20 | nd | < 0.50 | 32.78 |
| WBC (× 103/μL) | 6.1–12.0 | 0.7 | 0.6 | 3.7 | 10.1 | 7.2 |
| Segmented neutrophils (× 103/μL) | 4.0–8.2 | 0.4 | 0.1 | 2.3 | 8.8 | 5.3 |
| Lymphocytes (× 103/μL) | 0.8–3.6 | 0.4 | 0.1 | 1.0 | 1.0 | 1.4 |
| Clinical chemistry | ||||||
| Urea (mmol/L) | 1.8–12.1 | 15.7 | 4.6 | |||
| Albumin (g/L) | 28–40 | 23 | 21 | |||
| Globulins (g/L) | 22–41 | 17 | 21 | |||
| Bicarbonate (mmol/L) | 18–24 | 9 | 21 | |||
| Anion gap (mmol/L) | 12–22 | 28 | 17 | |||
| Calcium (mmol/L) | 2.34–2.72 | 1.5 | 2.4 | |||
| Phosphorus (mmol/L) | 0.68–1.48 | 2.13 | 2.5 | |||
| Ammonium (μmol/L) | 0–21 | 128 | ||||
| ALP (U/L) | 13–107 | 259 | ||||
| Serum cobalamin (ng/L) | 250–908 | <150 | ||||
| Serum folate (μg/L) | 7.7–24.4 | 3.6 | ||||
| Urine MMA (mol/mol creatinine) | < 0.034 | > 1.5 | ||||
5 months of age.
Initiated parenteral cobalamin administration.
nd — Not done.
Figure 1.
Selected peripheral blood film findings on days 1 (A–G), 3 (H–L), 5 (M–N), and 40 (O). At presentation, neutrophils were hypersegmented (A), most with mild diffuse cytoplasmic basophilia, and red blood cells variously exhibited echinocytosis (A–F), increased Howell-Jolly bodies (B), metarubricytes (C), short to long rectangular hemoglobin crystals (D), basophilic stippling (E), mildly polychromatophilic elliptocytes (F), and polychromatophilic erythrocytes, most of which were similar in diameter to mature erythrocytes (G). On day 3, diffusely basophilic hypersegmented neutrophils without Döhle bodies persisted (H and L); there were also low numbers of blasts (I) and mostly monocytoid cells with increased diffuse cytoplasmic basophilia (J). Some cells had broad but rarely band-shaped nuclei and diffusely basophilic cytoplasm containing additional irregular patches of more intense basophilia (K). On day 5, hypersegmented neutrophils were more numerous (M and N). Throughout, some hypersegmented neutrophils were large (M). With recovery, circulating neutrophils appeared as in health, lacking cytoplasmic basophilia and hypersegmentation (O). The scale bar in frame O applies to all frames. Routine Romanowsky stains: Wright-Giemsa (A–G, M–O) and Modified Wright (H–L).
A serum biochemical profile showed mild elevation of urea with mildly decreased creatinine, moderate panhypoproteinemia, a high anion gap metabolic acidosis, mild hyperphosphatemia, moderate hypocalcemia, and moderate hyperammonemia (Table 1). Urine pH and specific gravity were 6.4 and 1.038, respectively. There was cylindruria characterized by 10 to 15 hyaline casts and 4 to 6 granular casts per low power field. Urine ketones were > 80 mg/dL without glucosuria. Proteinuria was not detected by sulfosalycylic acid precipitation.
Thoracic and abdominal radiographs revealed mild generalized cardiomegaly, with possible left atrial enlargement, and a moderately distended stomach filled with food. Abdominal ultrasound examination confirmed the food-filled stomach, but no other abnormalities were observed. Echocardiogram was unremarkable.
The pup was admitted to the critical care unit, and treatment was initiated with balanced isotonic crystalloid solution (Lactated Ringer’s Injection USP; Hospira, Lake Forest, Illinois, USA), starting at 5.6 mL/kg body weight (BW) per hour and gradually reducing the rate over the stay in the hospital, ampicillin Na/sulbactam Na (Unasyn; AuroMedics Pharma, Dayton, New Jersey, USA), 30 mg/kg BW, IV, q8h, and a 1/4 unit of packed red blood cells. Repeat abdominal radiographs showed persistence of the gastric material, and prokinetic therapy was commenced with erythromycin (Erythrocin; Hospira), 0.5 mg/kg BW, IV, q8h. Forty hours after admission, the patient developed marked persistent pyrexia (rectal temperatures ranged from 39.5°C to 40.8°C), and a repeat CBC on day 3 showed persistent anemia and leukopenia (Table 1) with the same constellation of leukocyte and erythrocyte abnormalities that were present on day 1. Additionally, however, the predominating monocytoid cells had increased diffuse to patchy cytoplasmic basophilia such that they blended with the neutrophil population characterized by broad, mildly lobulated nuclei and diffuse cytoplasmic basophilia with patches of more intense basophilia (Figure 1). There were also few myeloblasts or monoblasts. Because of the presence of hypersegmented neutrophils and other leukocyte changes that were not typical of toxic change, an additional dyshematopoietic state was suspected. Antibacterial coverage was broadened with the addition of clindamycin (Pfizer, New York, New York), 10 mg/kg BW, IV, q12h, and ceftazidime (WG Critical Care, Paramus, New Jersey), 25 mg/kg BW, IV, q8h. Two blood samples were collected from different peripheral veins, 1 h apart, and submitted for aerobic and anaerobic bacterial culture and sensitivity but yielded no growth. Enteral nutrition was commenced through a nasogastric tube with feedings starting at one-quarter of the calculated resting energy requirement (Clinicare; Abbott Laboratories, North Chicago, Illinois, USA), and supplementation with thiamine (Sparkhawk Laboratories, Lenexa, Kansas, USA), was initiated at 50 mg IM, q12h. High dose parenteral therapy with cyanocobalamin (Sparkhawk Laboratories), 500 μg SQ q48h, was instituted on day 3, prior to laboratory confirmation of deficiency, due to suspicion of inherited selective cobalamin malabsorption (I-GS) based on the breed, age, CBC results, anorexia, and poor body condition of the patient.
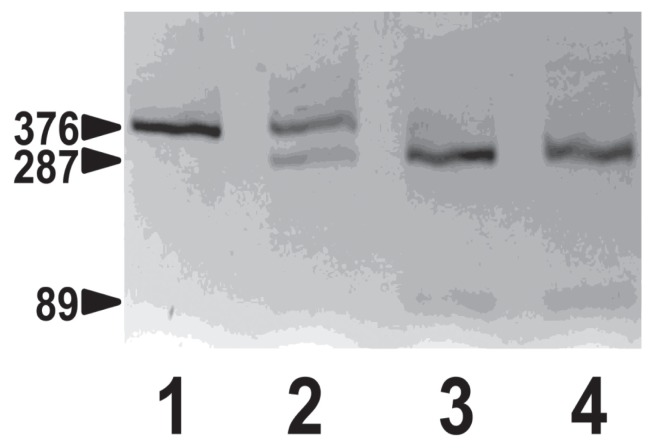
In a sample collected on day 1, prior to cobalamin supplementation, serum cobalamin concentration was undetectable (Table 1) and folate concentration was low. Methylmalonic acid (MMA) was > 40-fold higher than normal in pre-treatment urine (2). Serum homocysteine was not measured. The Australian shepherd amnionless (AMN) mutation site was amplified by polymerase chain reaction (PCR) from genomic DNA isolated from blood collected in ethylenediamine tetra-acetic acid (EDTA) anticoagulant using previously described methods (2). The patient was homozygous for the AMN c.3G > A mutation, thus providing definitive diagnosis of canine I-GS (Figure 2).
Figure 2.
Genotyping test for Australian shepherd AMN mutation. Shown are Bst F5 I restriction fragments of AMN exon 1 genomic DNA amplified by polymerase chain reaction separated by 3% agarose gel electrophoresis. Lane 1, the I-GS affected Australian shepherd dog of this report; lane 2, a clinically normal littermate; lane 3, a normal beagle; lane 4, a normal great Dane. The AMN c.3G > A mutation obliterates a Bst F5 I restriction site, thus leaving the PCR product amplified from the mutation allele intact at 376 base pair (bp) but cutting the product amplified from the normal allele into fragments of 89 and 287 bp.
Four days following the initial parenteral supplementation with cobalamin (day 7), the pup was bright and responsive, euhydrated, had a robust appetite, and had vital parameters within normal limits. Repeat hematologic testing revealed persistent normocytic, hypochromic, nonregenerative anemia with reticulocytopenia, but the leukopenia had resolved and there was a mild mature neutrophilia with few mildly basophilic hypersegmented neutrophils (Table 1). A rise in the neutrophil concentration was first detected 2 d earlier, the second day after cobalamin administration. The patient was discharged from MSU-VMC on broad-spectrum antibiotics (cefpodoxime proxetil (Simplicef; Zoetis, Kalamazoo, Michigan, USA), 6 mg/kg BW, PO, q12h, for 7 d and metronidazole (Watson Pharmaceuticals, Parsippany, New Jersey, USA) 10 mg/kg BW, PO, q12 for 7 d, and prokinetic therapy (Metoclopramide; Pharmaceutical Associates, Greenville, South Carolina, USA), 0.25 mg/kg BW, PO, q8 for 5 d.
On recheck 8 d later (day 15), the dog had gained 0.6 kg and was thriving at home, with excellent appetite and energy. Recheck CBC revealed a regenerating erythron (Table 1) with moderate polychromasia, white blood cell and neutrophil concentrations within reference limits, and a moderate thrombocytosis. A serum chemistry panel revealed a persistent age-expected hyperphosphatemia, mild hyperalkaline phosphatemia, and unresolved mild hypoalbuminemia and hypoglobulinemia. The dog was maintained on weekly cyanocobalamin injections (75 μg/kg BW, SC) for 1 mo and then transitioned to twice monthly injections (50 μg/kg BW, SC). The patient responded completely to cobalamin supplementation and now, 8 mo later, remains asymptomatic following cessation of all therapies other than parenteral cobalamin administration.
Discussion
Cobalamin (vitamin B12) is is required for normal growth and development, hematopoiesis, and nucleic acid synthesis (11,12). Monogastric animals are unable to synthesize cobalamin and are dependent on intestinal absorption of the vitamin from dietary sources primarily of animal origin. Cobalamin liberated from food binds first to haptocorrin, which delivers the vitamin to the duodenum. There cobalamin binds to intrinsic factor (produced by the stomach and pancreas in dogs), and the cobalamin-intrinsic factor complex undergoes receptor-mediated endocytosis in the distal ileum (13,14). The ileal receptor, known as cubam, is a complex of 2 subunits, the proteins amnionless (AMN) and cubilin (CUBN) (15–17). Once absorbed across the intestinal mucosa, cobalamin is transported in plasma throughout the body complexed to transcobalamin (18). As such, successful cobalamin absorption is a sequence of protein-binding events that each depend on the longitudinal secretory and absorptive organization of the GI tract. Therefore, cobalamin deficiency in dogs can have a variety of causes, including insufficient dietary cobalamin, pancreatic disease, infiltrative or inflammatory intestinal disease, intestinal dysbiosis, generalized malabsorptive disorders, or ileal resection (1).
Cobalamin is a cofactor in the metabolic pathways to convert methylmalonyl-CoA to succinyl-CoA and for remethylation of homocysteine to form methionine (11). Cobalamin deficiency reduces the activity of these enzymes, shifting metabolites to alternate pathways and resulting in increased plasma methylmalonic acid (MMA) and homocysteine concentrations (11). Methylmalonic acid is readily detected in urine because the renal tubule has no transporter for the abnormal metabolite. Methylmalonic acidemia/aciduria and homocysteinemia are classic findings in patients with cobalamin deficiency, although elevated MMA concentration is a more specific biomarker. Presumably, MMA and ketone bodies contributed to this dog’s high anion gap metabolic acidosis. Cubam also functions in renal proximal tubule epithelium to mediate reabsorption of specific mid- to low-molecular-weight proteins from glomerular filtrate (8,15). Therefore, abnormal cubam expression due to either CUBN or AMN mutation causes selectively increased proteins in urine, in addition to methylmalonic aciduria. The patient described herein had a highly elevated urine MMA concentration, but proteinuria was not detected by sulfosalicylic acid precipitation. However, excess proteins recognized as cubam ligands, including transferrin, albumin, lipoprotein apo A1, haptoglobin, and vitamin D-binding protein were observed by SDS-PAGE (not shown) (8).
The canine syndrome described here mimics human I-GS which is typified by selective cobalamin malabsorption with proteinuria. It was first described in 1960 in children of Finnish and Norwegian descent (9,10). I-GS affects the pediatric population, but the age of presentation varies from a few months to puberty. Treatment in humans involves monthly injections of 1 mg of cyanocobalamin or hydroxocobalamin for life (19). In humans it is a rare disorder typically occurring in familial or ethnic clusters. Approximately 400 cases have been reported worldwide to date, and mutations of CUBN or AMN have been documented in most (19).
Imerslund-Gräsbeck syndrome was first recognized in dogs in 1989 (20). Since that time several studies have documented the syndrome in giant schnauzers, border collies, beagles, and Australian shepherds as an autosomal recessive disorder in each breed (2,3,7,8). Dogs that inherit I-GS typically begin to manifest signs when fetal stores of cobalamin are depleted and growth demands are highest, at 8 to 12 wk of age. However, due to vague or complicated and nonspecific signs of disease, some affected dogs may not come to veterinary attention until months later. Hematologic abnormalities include dyshematopoiesis characterized by nonregenerative anemia with metarubricytosis but typically normal Wintrobe indices, and neutropenia with hypersegmented neutrophils and megaloblastic changes in hematopoietic precursors (2,3). Unlike their human cobalamin-deficient counterparts, dogs with this disorder do not develop macrocytic anemias, but instead typically have normocytic normochromic anemias (3). Similarly, the dog in this case had a nonregenerative normocytic anemia with mild metarubricytosis, as well as neutropenia and hypersegmented neutrophils, some of which were macrocytic. However, this dog’s anemia was persistently hypochromic, suggesting reduced hemoglobin synthesis and further impairment of erythropoiesis.
Hematopoiesis was not further characterized by bone marrow examination, as it was unnecessary for appropriate patient management, but additional peripheral blood abnormalities suggested further impairment of hematopoiesis in this dog. The smaller than usual polychromatophilic erythrocytes that were present in pre-treatment blood along with Howell-Jolly bodies, metarubricytosis, basophilic stippling, and intra-erythrocytic hemoglobin crystals suggested an altered and inadequate erythropoietic response due to cobalamin and possibly other secondary nutrient deficiencies. In addition to the large and hypersegmented neutrophils indicative of dysmyelopoiesis, most pre-treatment neutrophils had diffuse cytoplasmic basophilia, and, after pyrexia developed, neutrophils and monocytes had atypical nuclear or cytoplasmic features that made their identification and differentiation difficult. Some of these changes could have been induced by inflammation given the pyrexia at that time, but the aggregates of basophilia in monocytes, the more monocytoid shape and chromatin pattern of neutrophil nuclei, the patchy basophilia rather than distinct Döhle bodies in neutrophils, and the presence of low numbers of unidentified blasts were not typical inflammatory changes.
In addition to the hematologic changes outlined and methylmalonic aciduria, affected dogs can also exhibit secondary metabolic derangements. The primary metabolic defects caused by cobalamin deficiency secondarily inhibit the urea cycle and gluconeogenesis. Affected dogs may exhibit hypoglycemia, ketoacidosis, and hyperammonemia (3–8), all but hypoglycemia having been present in this dog. Affected dogs typically present with a history of chronic lethargy, inappetence, failure to gain weight, vomiting, diarrhea, or seizures. Because of the nonspecific clinical signs, patients may be misdiagnosed or suspected to have other diseases causing poor thrift. Differential diagnoses that should be considered include congenital abnormalities (such as portosystemic shunts), infectious diseases (toxoplasmosis, neosporosis, distemper, parvoviral infection, intestinal parasitism), congenital hypothyroidism, toxin ingestion, thiamine deficiency, or other inborn errors of metabolism. Breed predisposition to the various inherited disorders should always be considered. In the present case, the clinical picture fit most closely with cobalamin malabsorption of Australian shepherds, and response to cobalamin supplementation was so complete that ruling out many of the above-mentioned diseases was not necessary.
Genetic studies have determined the exact mutations causing defective cobalamin absorption in the aforementioned dog breeds. Mutations in CUBN cause I-GS in border collies and beagles, and mutations in AMN cause I-GS in giant schnauzers and Australian shepherds (2,7,8). Genetic tests are available for each breed (http://research.vet.upenn.edu/WSAVA-LabSearch is a searchable compendium of laboratories performing genetic tests for dogs and cats). While I-GS is uncommon among all dogs, in the affected breeds there can be a substantial carrier frequency and more common occurrence of disease regionally or worldwide (7,8). Affected dogs of the various breeds have been reported from Canada, USA, UK, continental Europe, and Australia. In affected Australian shepherds, a breed that was developed exclusively in western North America, rather than Australia as the name suggests, a G > A transition at position 3 of cDNA (c.3G > A) was identified that abrogates translation of the AMN protein (2). Without AMN, the cubam receptor is not expressed in the epithelial brush border, and there is no absorption of dietary cobalamin from the ileum. The disease trait is recessive, and only dogs homozygous for the mutant allele are symptomatic (2). DNA collected from the patient reported here confirmed that she was homozygous for the c.3G > A AMN mutation. DNA from a clinically normal male littermate was heterozygous for the AMN mutation (Figure 1). DNA and information on the remainder of family members was unavailable, but it is clear that both parents of the affected dog are obligate carriers.
Treatment of I-GS in dogs requires parenteral administration of cyanocobalamin. Initial doses of 75 μg/kg BW subcutaneously once weekly are recommended to reverse the deficiency state (1). Lifelong administration of cobalamin is also required to maintain normal metabolism, but intervals between doses (50 μg/kg) of 3 to 4 wk can be sufficient (3,12). Cyanocobalamin has a wide margin of safety, and any excess is rapidly cleared through the kidneys (7). In all the dog breeds in which I-GS has been described, once cobalamin supplementation commences, anorexia and lethargy are expected to resolve within 1 to 2 d. Evidence of regeneration within the bone marrow is expected by 10 to 14 d, and urinary MMA concentrations should normalize within 7 d (3,12). In the dog described here, evidence of reticulocytosis was present 12 d following the first cyanocobalamin injection, having developed some days after resolution of neutropenia, which occurred between days 5 and 12 after treatment began. Supplementing cobalamin parenterally should abate any clinical signs, but subclinical proteinuria will persist lifelong due to abnormal cubam function in the kidneys that is not corrected by exogenous cobalamin supplementation (2,3,8).
This case report describes the clinical manifestations and treatment of selective intestinal cobalamin malabsorption confirmed by DNA testing in an Australian shepherd dog. The patient presented with clinical signs of weakness, inappetence and failure to grow, and hematologic abnormalities suggestive of dyshematopoiesis. Clinicians faced with anemic, neutropenic, and failing-to-thrive puppies, especially of the aforementioned breeds, should have a high index of suspicion for cobalamin malabsorption disorders. Finding low serum cobalamin concentrations, the presence of MMA in urine, and detecting the previously described gene mutations confirm the diagnosis. Swift and continued supplementation with parenteral cobalamin is vital to prevent a lethal metabolic crisis and ensure long-term survival in this easily treated disorder. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ruaux CG. Cobalamin in companion animals: Diagnostic marker, deficiency states and therapeutic implications. Vet J. 2013;196:145–152. doi: 10.1016/j.tvjl.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 2.He Q, Madsen M, Kilkenney A, et al. Amnionless function is required for CUBN brush-border expression and intrinsic factor-cobalamin (vitamin B12) absorption in vivo. Blood. 2005;106:1447–1453. doi: 10.1182/blood-2005-03-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fyfe JC, Giger U, Hall CA, et al. Inherited selective intestinal cobalamin malabsorption and cobalamin deficiency in dogs. Pediatr Res. 1991;29:24–31. doi: 10.1203/00006450-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Morgan LW, McConnell J. Cobalamin deficiency associated with erythroblastic anemia and methylmalonic aciduria in a border collie. J Am Anim Hosp Assoc. 1999;35:392–395. doi: 10.5326/15473317-35-5-392. [DOI] [PubMed] [Google Scholar]
- 5.Battersby IA, Giger U, Hall EJ. Hyperammonaemic encephalopathy secondary to selective cobalamin deficiency in a juvenile Border collie. J Small Anim Pract. 2005;46:339–344. doi: 10.1111/j.1748-5827.2005.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 6.Lutz S, Sewell AC, Reusch CE, Kook PH. Clinical and laboratory findings in border collies with presumed hereditary juvenile cobalamin deficiency. J Am Anim Hosp Assoc. 2013;49:197–203. doi: 10.5326/JAAHA-MS-5867. [DOI] [PubMed] [Google Scholar]
- 7.Fyfe JC, Hemker SL, Venta PJ, Stebbing B, Giger U. Selective intestinal cobalamin malabsorption with proteinuria (Imerslund-Gräsbeck Syndrome) in juvenile beagles. J Vet Intern Med. 2014;28:356–362. doi: 10.1111/jvim.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fyfe JC, Hemker SL, Venta PJ, et al. An exon 53 frameshift mutation in CUBN abrogates cubam function and causes Imerslund-Gräsbeck syndrome in dogs. Mol Genet Metab. 2013;109:390–396. doi: 10.1016/j.ymgme.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imerslund O. Idiopathic chronic megaloblastic anemia in children. Acta Paediatr Scand. 1960;49:1–115. [PubMed] [Google Scholar]
- 10.Gräsbeck R, Gordin R, Kantero I, Kuhlback B. Selective vitamin B12 malabsorption and proteinuria in young people. Acta Med Scand. 1960;167:289–296. doi: 10.1111/j.0954-6820.1960.tb03549.x. [DOI] [PubMed] [Google Scholar]
- 11.Markle HV. Cobalamin. Crit Rev Clin Lab Sci. 1996;33:247–356. doi: 10.3109/10408369609081009. [DOI] [PubMed] [Google Scholar]
- 12.Fordyce HH, Callan B, Giger U. Persistent cobalamin deficiency causing failure to thrive in a juvenile beagle. J Small Anim Pract. 2000;41:407–410. doi: 10.1111/j.1748-5827.2000.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 13.Marcoullis G, Rothenberg SP. Intrinsic factor-mediated intestinal absorption of cobalamin in the dog. Am J Physiol. 1981;241:294–299. doi: 10.1152/ajpgi.1981.241.4.G294. [DOI] [PubMed] [Google Scholar]
- 14.Batt RM, Horadagoda NU. Gastric and pancreatic intrinsic factor-mediated absorption of cobalamin in the dog. Am J Physiol. 1989;257:G344–G349. doi: 10.1152/ajpgi.1989.257.3.G344. [DOI] [PubMed] [Google Scholar]
- 15.Fyfe JC, Madsen M, Hojrup P, et al. The functional cobalamin (vitamin B12)-intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood. 2004;103:1573–1579. doi: 10.1182/blood-2003-08-2852. [DOI] [PubMed] [Google Scholar]
- 16.Aminoff M, Carter JE, Chadwick RB, et al. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat Genet. 1999;21:309–313. doi: 10.1038/6831. [DOI] [PubMed] [Google Scholar]
- 17.Tanner SM, Aminoff M, Wright FA, et al. Amnionless, essential for mouse gastrulation, is mutated in recessive hereditary megaloblastic anemia. Nat Genet. 2003;33:426–429. doi: 10.1038/ng1098. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee R. B12 trafficking in mammals: A case for coenzyme escort service. ACS Chemical Biology. 2006;1:149–159. doi: 10.1021/cb6001174. [DOI] [PubMed] [Google Scholar]
- 19.Gräsbeck R, Tanner SM. Juvenile selective vitamin B12 malabsorption: 50 years after its description — 10 years of genetic testing. Pediatr Res. 2011;70:222–228. doi: 10.1203/PDR.0b013e3182242124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fyfe JC, Jezyk PF, Giger U, Patterson DF. Inherited selective malabsorption of vitamin B12 in giant schnauzers. J Am Anim Hosp Assoc. 1989;25:533–539. [Google Scholar]