Abstract
Objective
Cancer risk-related stressors are prominent among BRCA mutation carriers. Loss of one’s mother to a BRCA-associated cancer is an additional stressor, which may be related to an enhanced inflammatory response. This study examined the effect of mother’s vital status on psychological factors and stress-associated biomarkers among BRCA mutation carriers. The role of bereavement on biopsychological variables was also examined.
Methods
BRCA-carriers with known maternal transmission enrolled in the Gilda Radner Hereditary Cancer Program were invited to participate. Focus group composition was predetermined based on participants’ personal cancer history and mother’s vital status. Prior to the focus group, participants completed a Quality of Life (QOL) survey and collected a first morning saliva sample. Inflammatory biomarkers were analyzed from proximal archived serum. One day post focus group, a process survey, and morning saliva were collected.
Results
QOL was significantly lower for those whose mothers are deceased (n = 17) compared to those whose mothers are alive (n = 15) (P = 0.003) after adjusting for age, personal cancer history and prophylactic surgery. Similarly, those whose mothers are deceased reported significantly more perceived stress (P = 0.015), more intrusive thoughts related to cancer risk (P = 0.049), and more anxiety (P = 0.003). Higher bereavement scores were significantly associated with QOL and psychological measures. Biomarker correlates were consistent with and significantly correlated to the patient-reported psychological outcomes for those whose mothers were deceased.
Conclusions
BRCA mutation carriers with a known maternal transmission whose mother is deceased report higher perceived stress and anxiety, lower QOL, and a stress-associated biomarker profile that is potentially globally immune suppressive.
Literature indicates that symptoms of anxiety, depression, and stress related to cancer risk can be prominent among women with a family history of breast cancer and among breast cancer (BRCA)-mutation carriers.1–7 Although it is recognized that women with BRCA mutations cope well with health information and decision-making, it does not negate the fact that this information adds a unique psychological, social, and health-specific burden. Decision-making around management of health concerns and cancer surveillance is a critical feature of care, which can serve to both relieve8–12 and intensify stress.13–16 An additional somewhat unique stressor to this population is that of mother’s death from cancer. Zakowski and colleagues have identified that women who experienced a mother’s death from cancer suffer particularly severe or prolonged difficulties adjusting to the loss.17 Previous research has also noted that women with family histories of breast cancer whose mothers had died of the disease experienced higher levels of distress than family history positive women whose mothers had not died of breast cancer, or family history negative women.4 Similarly, loss of a parent in childhood, adolescence, or early adulthood negatively affects psychosocial and physiological well-being.18–22 Taken together, this body of literature supports further consideration of vulnerable subpopulations of women at high risk of cancer, thereby leading to improved care and health outcomes.
The literature on grief and bereavement provides an important avenue to further this discussion, indicating that while this circumstance is encountered by most individuals during their lifespan, there are conditions in which grief may exact a chronic psychological and physiological toll.23,24 These conditions include having been a child of a parent with cancer,6,25 being female,23,26 and having had an early experience of a parental death.7,18,27 Although this literature has been substantiated over many years, to our knowledge the topic of grief and bereavement has not specifically been linked to biopsychological risk factors for chronic stress among BRCA mutation carriers, despite the fact that a majority of carriers will have lost a parent, and other relatives, to cancer. Furthermore, there is abundant literature indicating that people with high rates of life stress exhibit enhanced inflammatory responsiveness,18,28 – 30 which may play a role in disease penetrance.27
Therefore, the purpose of this pilot study was to further characterize potentially vulnerable subpopulations of BRCA mutation carriers by hypothesizing that: (1) mother’s vital status will be related to BRCA mutation carriers perceived stress, quality of life, and distress; (2) perceived stress, quality of life, and distress will be related to level of bereavement, and (3) stress-associated biomarkers will be related to perceived stress. If validated, these findings could lead to specific stress and/or inflammation reducing interventions that may reduce cancer penetrance in this high risk population.
METHODS
Study Participants
Under Institutional Review Board-approved protocol (18941) women with BRCA 1/2 mutations were identified through the Gilda Radner Hereditary Cancer Program at Cedars-Sinai Medical Center. Eligibility was limited to those participants over the age of 18 with maternal transmission of the mutant BRCA gene and whose mothers were affected and previously diagnosed with ovarian, peritoneal, fallopian tube, and/or breast cancers, and who themselves were in remission for cancer or did not have a cancer history (n = 112). Those eligible were also required to indicate a willingness to participate in a focus group designed to solicit feedback for future program planning.
Pre-Focus Group
Initial verbal consent was obtained during recruitment, with subsequent written consent. Enrolled participants were sent a questionnaire packet and a saliva collection kit via Federal Express approximately 10 days prior to the date of the scheduled focus group. Participants were asked to complete the questionnaire and provide a first morning saliva sample within 48 hours after the completion of the survey. Saliva specimens were returned to the study office in a cooler with ice packs within 24 hours, via Federal Express, along with the survey.
MEASURES
In addition to general medical history, family history, and basic demographic information, questionnaires specific to quality of life, stress, distress, and bereavement were selected based on psychometric properties and potential applicability to this population.
Quality of Life
The Functional Assessment of Cancer Therapy-General (FACT-G) quality of life was utilized to assess the 4 domains of physical well-being, social/family well-being, emotional well-being, and functional well-being. This instrument has been used to assess overall quality of life for those with and without specific illnesses. For our purpose, 5 questions directly related to illness were removed from the survey, which is consistent with a validated approach to assessing QOL in healthy populations.31
Perceived Stress
The perceived stress scale (PSS) is a 10-item scale that measures the degree to which life situations are appraised as stressful and to what degree participants feel that their lives are unpredictable, uncontrollable, and overloaded with responsibility.32
Disease-Specific Stress
The impact of events scale (IES) is a 15-item questionnaire that measures intrusive feelings and thoughts about breast/ovarian cancer risk, and avoidance of these feelings, and thoughts.33
Emotional Distress
The patient-reported outcomes measurement information system (PROMIS) (www.NIHPROMIS.org) Depression and Anxiety short forms were used. The PROMIS item bank for depression focuses how negative mood impacts the individual’s feelings of worthlessness. The item bank for anxiety examines fear and the constant feeling of worry.
Bereavement
The core bereavement items (CBI) questionnaire consists of three subscales originating from the 76-item bereavement phenomenology questionnaire.34 This modified version has demonstrated both reliability and validity in multiple populations, including bereaved adult children.
Biomarkers
Morning salivary cortisol samples were collected to identify potential associations between patient-reported stressors related to living with BRCA-associated cancer risk, and stress-associated salivary cortisol and dehydroepiandrosterone (DHEA) levels. Participants were instructed to collect salivary samples upon awakening and before brushing their teeth. This highly motivated population reported the collection time for salivary samples that were consistent with the provided instructions. The timing of these collections is noted above. In addition, proximal archived serum samples were analyzed for select cytokines to explore the relationship between the stress reported in surveys, the stress levels identified in DHEA, and salivary cortisol levels, and potential stress associated immune perturbation. All serum samples were obtained within 6 months of the focus groups with the assumption that levels of stress associated with BRCA-associated cancer risk, maternal cancer diagnosis, and/or maternal death, were unlikely to have changed significantly over this time period because of the absence of new events in and immediately proximal to this 6-month time period.
Focus Group Composition and Conduct
Consenting women were assigned to inclusion in one of the following predetermined focus groups: group 1: BRCA positive, maternal transmission, no prior cancer diagnosis, mother diagnosed with cancer, mother deceased (n = 12), group 2: BRCA positive, maternal transmission, no prior cancer diagnosis, mother diagnosed with cancer, mother alive (n = 14); group 3: BRCA positive, maternal transmission, prior cancer diagnosis not under active treatment, mother diagnosed with cancer, mother deceased (n = 5); group 4: BRCA positive, maternal transmission, prior cancer diagnosis, mother diagnosed with cancer, mother alive (n = 1). Group 4, those with prior cancer diagnosis whose mothers were alive were combined with those with a cancer diagnosis whose mothers were deceased due to small numbers in each group. The a priori group differentiation was justified based on hypotheses that there may be differences in how women respond to questions based on their personal and familial experience with cancer. Answers to open-ended questions were solicited in order to obtain thoughts and opinions on topics related to stressors affecting quality of life for BRCA 1/2 mutation carriers, and obtain feedback on dimensions of health care and program planning which could be revised or improved.
Post-Focus Group Survey and Biomarker Collection
After the focus group, participants were given a brief process survey, designed to obtain opinions on the extent to which focus group participation was potentially helpful or stressful. They also received a saliva collection kit to take home. Participants were asked to complete the survey on the same day as the focus group, and to provide a saliva specimen in an identical manner to the first collection, within 48 hours of completion of the survey. The survey and saliva specimens were returned to the study office via Federal Express and the saliva was shipped at approximately 4 °C.
Statistical Analyses
Quantitative questionnaire data were analyzed through t-test for group comparisons, and multivariate linear models to test for differences between groups after adjusting for covariables. Pearson and Spearman correlations were conducted to determine relationships between measures of pre-focus group emotional distress, post-focus group stress and cortisol and biomarkers in archived serum samples.
RESULTS
Recruitment Characteristics
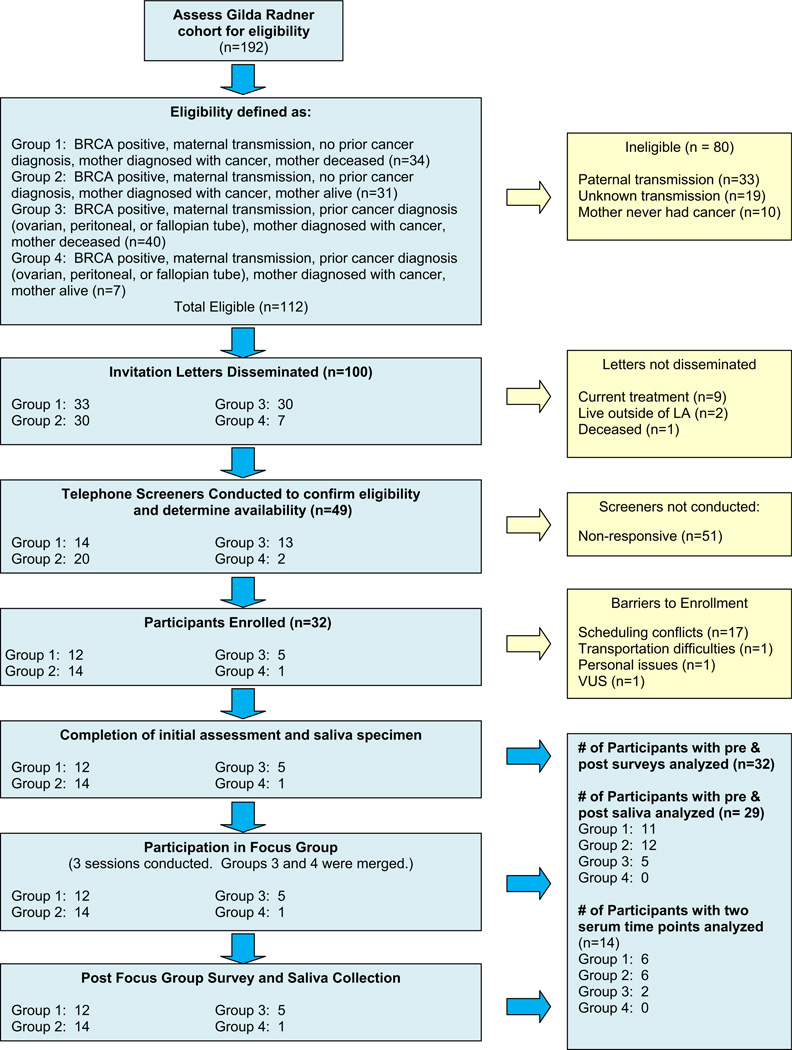
In 2009, 192 BRCA mutation carriers enrolled in the Gilda Radner Hereditary Cancer Program were initially identified as eligible to participate in this study. Of those, 100 were eligible and were invited to participate. Ninety-two were ineligible due to identified paternal transmission (n = 33), unknown transmission (n = 19), mother never had cancer (n = 10), undergoing cancer treatment (n = 9), residing outside of Los Angeles (n = 2), or deceased (n = 1). As indicated in Figure 1, a telephone screening process for prospective participants yielded 49 women who were interested, with 32 available during the defined study period, providing an overall 32% participation rate for those eligible and invited.
FIGURE 1.
Recruitment Flowchart
Sociodemographic Characteristics
The majority of eligible patients were non-Hispanic white (>88%), of Ashkenazi Jewish descent, and between the ages of 29 and 74 (mean age 48). The majority (81%) did not have a personal cancer history; 66% had undergone a prophylactic bilateral salpingo-oophorectomy (BSO); and 19% had undergone a prophylactic mastectomy. Those with a personal cancer history were significantly older than those without (P < 0.001). Among those whose mothers were deceased (n = 17), their age at mother’s death ranged from 3 to 48 years, with a mean of 32 years (Table 1).
TABLE 1.
Descriptive Table
| n | Mean | SD | Range | |
|---|---|---|---|---|
| Age at Focus Group | 32 | 48.6 | 12.7 | 29–74 |
| Age at BRCA test | 27 | 43.1 | 12.9 | 23–72 |
| Age at mother’s death | 16 | 32.1 | 12.5 | 3–48 |
| FACT-G | 31 | 87.0 | 17.1 | 37–106 |
| Perceived stress | 31 | 16.5 | 7.1 | 2–32 |
| Emotional distress-total | 32 | 32.1 | 11.0 | 15–56 |
| Bereavement total score | 17 | 19.4 | 10.2 | 3–43 |
| n | % | |||
| Personal history of cancer | ||||
| Yes | 6 | 19 | ||
| No | 26 | 81 | ||
| Mother deceased | ||||
| Yes | 17 | 53 | ||
| No | 15 | 47 | ||
| Prophylactic BSO | ||||
| Yes | 21 | 66 | ||
| No | 11 | 34 | ||
| Prophylactic mastectomy | ||||
| Yes | 6 | 19 | ||
| No | 26 | 81 | ||
| Ethnicity | ||||
| Caucasian | 28 | 88 | ||
| African-American | 1 | 3 | ||
| Latina | 3 | 9 | ||
| Education | ||||
| High School/some college | 6 | 19 | ||
| College graduate | 8 | 26 | ||
| Graduate/professional | 17 | 55 | ||
| Income | ||||
| <$50 K | 4 | 14 | ||
| $50 K–$100 K | 7 | 24 | ||
| $100 K–$150 K | 7 | 24 | ||
| >$150 K | 11 | 38 | ||
| Marital status | ||||
| Single | 8 | 26 | ||
| Married | 17 | 55 | ||
| Divorced | 4 | 13 | ||
| Widowed | 2 | 6 |
BRCA = breast cancer; FACT-G = Functional Assessment of Cancer Therapy-General; BSO = bilateral salpingo-oophorectomy.
Perceived Stress, Distress, and Quality of Life
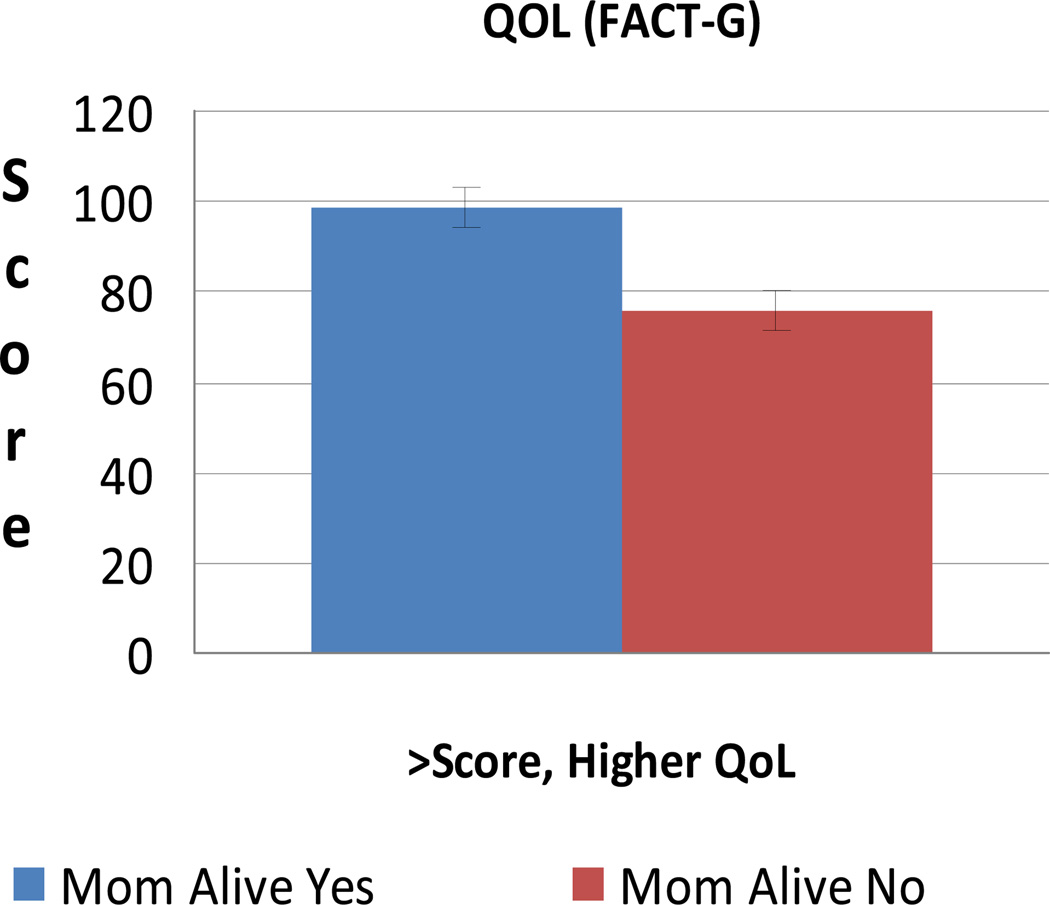
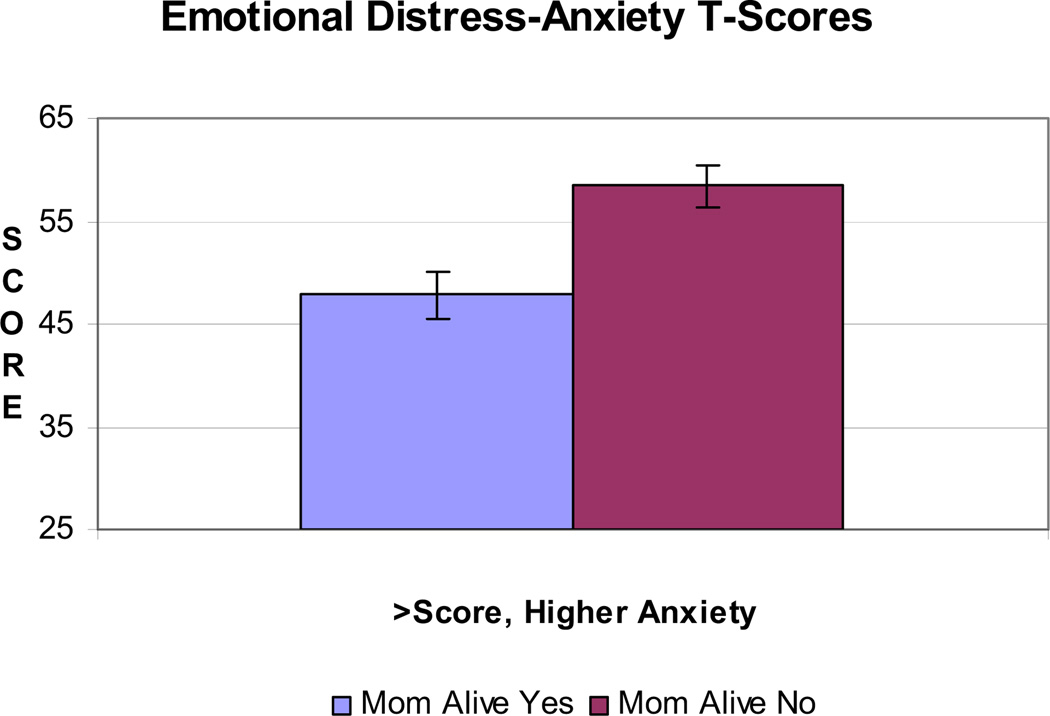
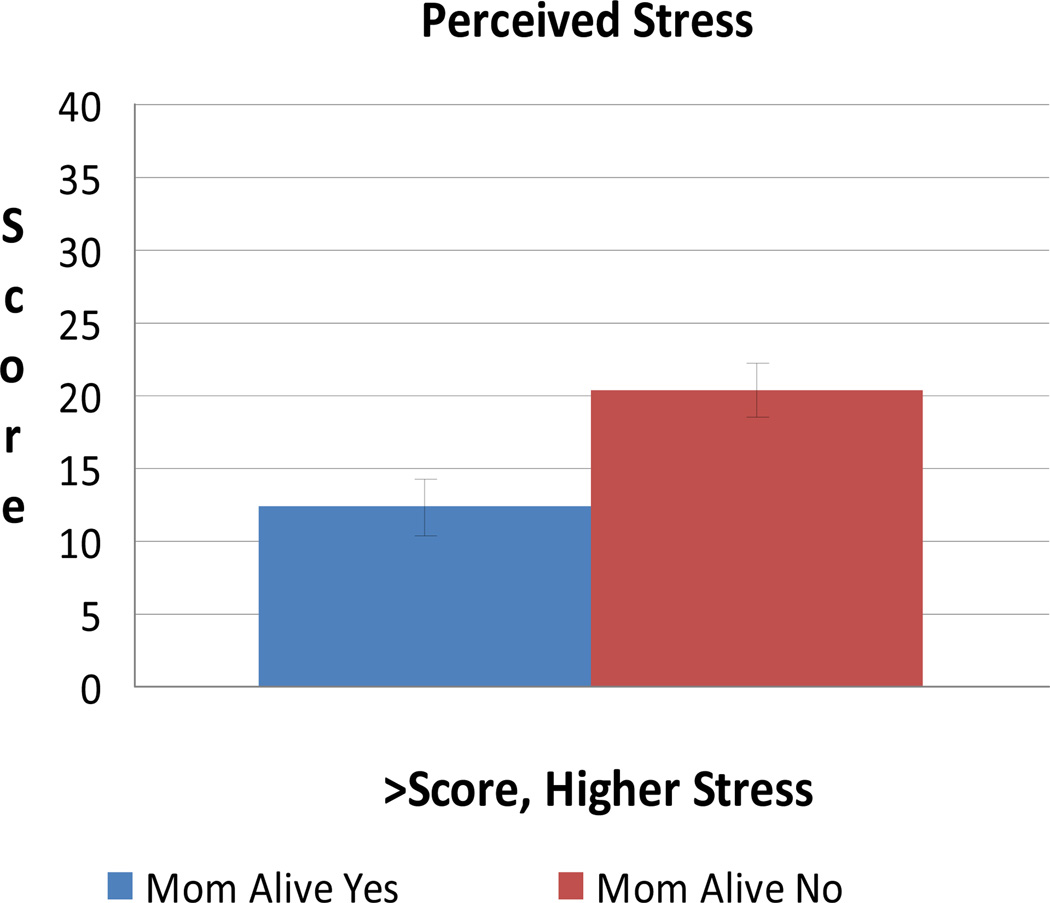
After adjusting for age, personal cancer history, and prophylactic surgery, quality of life is significantly lower for those whose mothers are deceased (n = 17) compared with those whose mothers are alive (n = 15) (P = 0.003) (Figure 2). Similarly, those whose mothers are deceased reported significantly more perceived stress (P = 0.015), more intrusive thoughts related to cancer risk (P = 0.049), and more anxiety (P = 0.003) (Figures 3 and 4). Depression was higher although non-significant (P = 0.12).
FIGURE 2.
Mean QOL (FACT-G)
FIGURE 3.
Mean Emotional Distress-Anxiety T-Score
FIGURE 4.
Mean Perceived Stress
Relationship to Bereavement
Lower quality of life and higher bereavement scores were significantly associated (r = −0.78, P < 0.001). Correlations were similarly strong for psychological measures of depression, anxiety, illness-specific intrusion, and perceived stress and their relationship to bereavement (r = 0.52, r = 0.49, r = 0.54, and r = 0.67 respectively). Bereavement scores were not correlated with the age of the participant at the time of mother’s death (r = 0.05, P = 0.84), but were related to the proximity of mother’s death (r = −0.45, P = 0.08) (higher scores with more recent death).
Biomarker Correlates
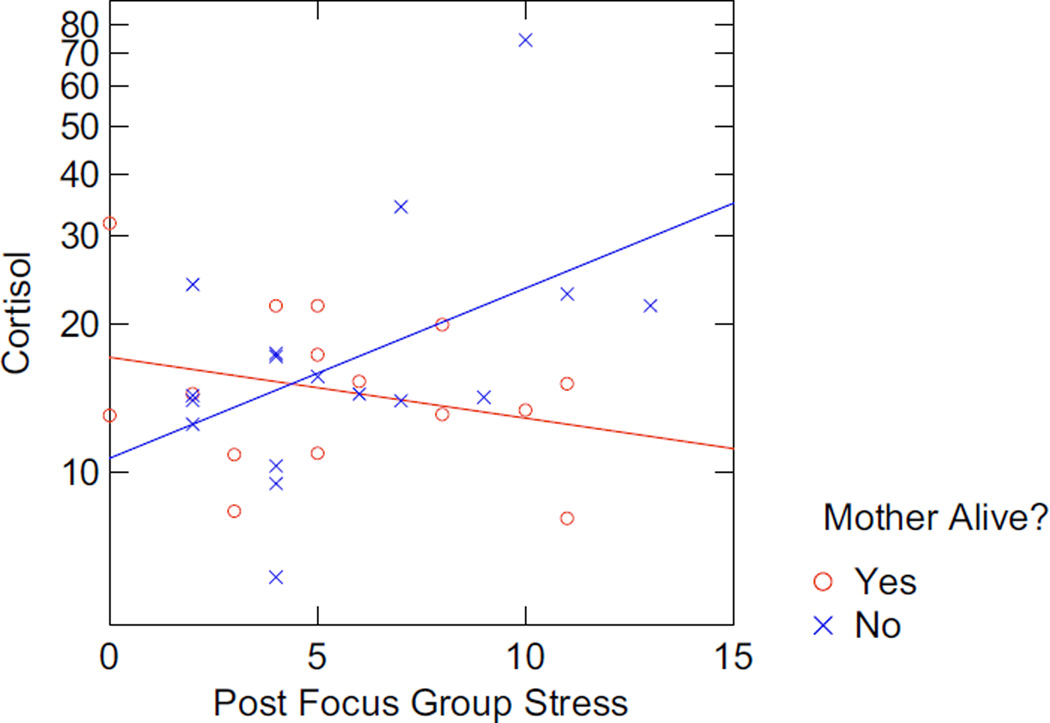
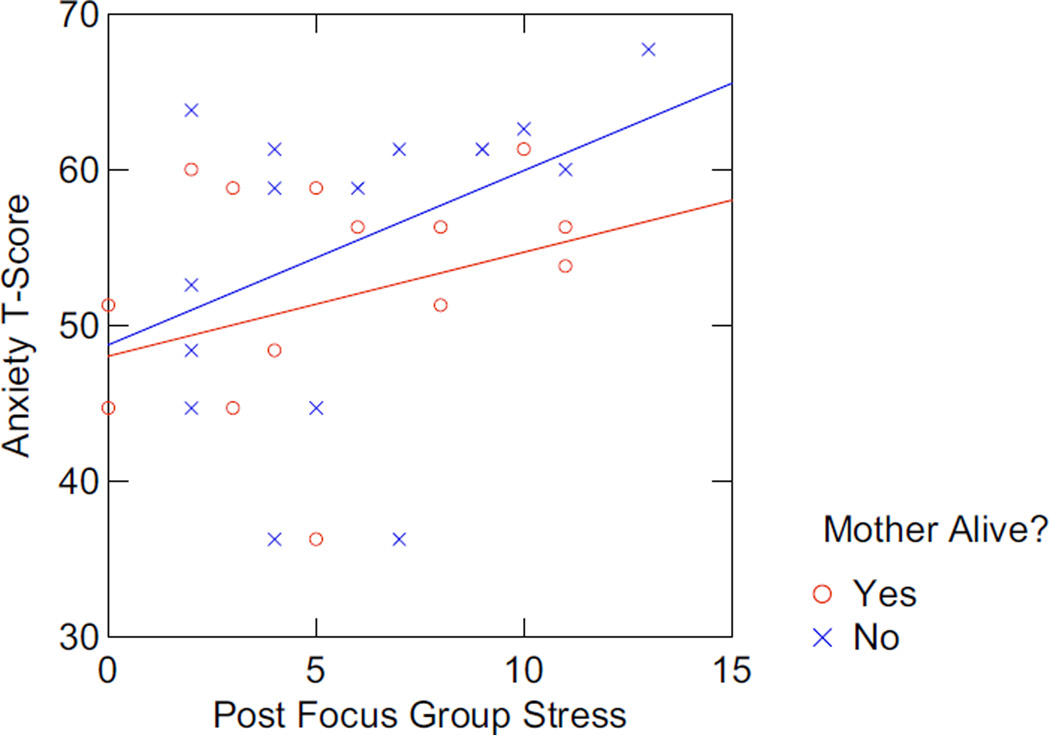
Biomarker associations with the patient-reported outcomes were most robust for those whose mothers were deceased and support our hypothesis that these stressors drive a more prominent pro-inflammatory immunologic stance. In this population, pro-inflammatory cytokine profiles were increased for those whose mothers were deceased (e.g., IL-17 associated with higher bereavement scores (r = −0.88; P = 0.023); tumor necrosis factor alpha (TNF-α) associated with QOL (r = 0.87, P = 0.05) and depression (r = −0.97, P = 0.03). In addition, the women who were most likely to report the highest levels of depression and anxiety and the lowest quality of life exhibited a not statistically significant trend toward higher cortisol levels, thereby providing support for these patient-reported outcomes (PROs) identifying individuals with higher chronic stress responses. Consistent with pre-focus group data, notably among those whose mothers are deceased, there is a correlative trend between post-focus group stress, anxiety (r = 0.40, P = 0.13), and salivary cortisol (r = 0.44; P = 0.09) (i.e., those who reported greater stress as a result of participating in the focus group also had higher anxiety scores, and higher cortisol) (Figures 5 and 6).
FIGURE 5.
Association Between Cortisol and Post Focus Group Stress
FIGURE 6.
Association Between Anxiety and Post Focus Group Stress
DISCUSSION
Previous research has noted that women with family histories of breast cancer whose mothers had died of the disease experienced higher levels of distress than family history positive women whose mothers had not died of breast cancer, or family history negative women.4,17 Prior studies did not, to our knowledge, purposefully select only women with a known BRCA 1/2 mutation with a known maternal transmission. Therefore, this study provides further justification to examine mechanisms by which this high risk carrier population may benefit from more tailored health care.
In our study, it was hypothesized that among BRCA mutation carriers with a known maternal transmission, perceived stress, distress, and quality of life would be associated with mother’s vital status, and may also be associated with stress-associated biomarkers. Indeed, after adjusting for characteristics known to be associated with quality of life, stress, and distress in a mutation carrier population, the factor most strongly associated with perceived stress, distress, and quality of life is mother’s vital status. This finding, while supporting aforementioned literature, adds a level of complexity to the understanding of stressors encountered by women who are BRCA mutation carriers, and could influence how, and for whom, counseling and medical decision making aids are provided.
It is an interesting and potentially novel finding that our measures of stress, distress, and quality of life were so highly correlated with bereavement scores. This may provide some confirmation that the death of Mom due to cancer has a powerful, long-standing impact on the carrier’s emotional well-being. This also begs the question, “In addition to psychological factors, might this generalize to biological factors associated with a heightened physical stress response?” Indeed, the biomarker correlates were consistent with the patient-reported outcomes only for those whose mothers were deceased, in that a general dampening of certain immune parameters was evident. Similarly, trends in post-focus group cortisol analyses, although not statistically significant, also suggested that those who reported greater stress as a result of participating in the focus group also had higher anxiety scores, and higher cortisol. This is worth examining in a larger confirmatory study since it also underscores the potential vulnerability of this population, and the impact these immune alterations could have on cancer penetrance or other stress-related health disorders.
For example, a recent study illustrates the prominent role of the adaptive arm of the immune system in cancer control and prognosis.35 In addition, proinflammatory states have been associated with increased risk of developing a number of pathologic processes including, but not limited to, atherosclerotic cardiovascular disease and cancer.36,37 Over the past several decades, the relationship between stress, inflammation, and health has been characterized most frequently by evaluation of interleukin 6 (IL-6) and C reactive protein (CRP) levels. IL-6 is produced by monocytes and CRP primarily in the liver in response to inflammatory cytokines including prominently IL-6, thereby reflecting activation of the innate arm of the immune system. Associations of stress with cytokines more reflective of the adaptive arm have been evaluated and reported particularly over the past decade typically featuring TNF-α,38–40 which has also been documented to play a role in carcinogenesis.41–43 There is an increasing appreciation of the complexity of the adaptive arm of the immune system and the role of IL-17 in many inflammatory pathophysiologic processes.44
We recognize that there are other hypotheses under investigation by multiple groups and that the relationships between various classes of immune responses and individual pathophysiologic processes are likely to be very complex.45,46 In this pilot study, although other cytokines characterizing different T helper classes were evaluated, there were no significant associations with cohort or questionnaire data, possibly due to limitations of sample size and methodology. Future studies will require more stringent confirmation of biospecimen collection that should be prospective and longitudinal, thereby limiting the need for use of archived biospecimens. Nevertheless, the associations described herein are pertinent to the hypothesis of chronic stress associated with pro-inflammatory states that could influence patient outcomes.
Further study limitations include the homogeneous sociodemographic characteristics, which are not representative of all mutation carriers. Specifically, this highly educated, Caucasian sample may have unique perceptions of life stress, and illness-specific stress, which may not necessarily be shared or experienced by less educated or minority populations. Therefore, it is reasonable to explore further how psychological, social, and health outcome vulnerabilities manifest in heterogeneous mutation carrier populations in order to build on previous biobehavioral studies. For example, a randomized psychosocial telephone counseling intervention improved symptoms of depression, anxiety, and genetic testing distress in a carrier population, compared with those in standard genetic counseling,47 and a nonrandomized supportive-expressive group therapy intervention improved cancer worries, anxiety, and depression.9 Although our pilot study did not account for influences of preexisting depression or anxiety on current stress, coping, or bereavement, larger longitudinal studies could easily incorporate this information. In turn, future studies could test a tailored care approach for mutation carriers at highest risk of deleterious health outcomes, particularly targeting those who lost their mother to cancer and/or report highest stress and distress.
Footnotes
Disclosure: The authors disclosed no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Lerman C, Daly M, Sands C, Balshem A, Lustbader E, Heggan T, et al. Mammography adherence and Psychological distress among women at risk for breast-cancer. J Natl Cancer I. 1993;85(13):1074–1080. doi: 10.1093/jnci/85.13.1074. [DOI] [PubMed] [Google Scholar]
- 2.Lerman C, Schwartz M. Adherence and psychological adjustment among women at high-risk for breast-cancer. Breast Cancer Res Tr. 1993;28:145–155. doi: 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- 3.Baider L, Ever-Hadani P, De-Nour AK. Psychological distress in healthy women with familial breast cancer: like mother, like daughter? Int J Psychiatry Med. 1999;29:411–420. doi: 10.2190/LD2F-ND7R-19JK-WL4G. [DOI] [PubMed] [Google Scholar]
- 4.Erblich J, Bovbjerg DH, Valdimarsdottir HB. Looking forward and back: distress among women at familial risk for breast cancer. Ann Behav Med. 2000;22:53–59. doi: 10.1007/BF02895167. [DOI] [PubMed] [Google Scholar]
- 5.Valdimarsdottir HB, Bovbjerg DH, Kash KM, Holland JC, Osborne MP, Miller DG. Psychological distress in women with a familial risk of breast-cancer. Psychooncology. 1995;4:133–141. [Google Scholar]
- 6.Mosher CE, Danoff-Burg S. Psychosocial impact of parental cancer in adulthood: A conceptual and empirical review. Clin Psychol Rev. 2005;25:365–382. doi: 10.1016/j.cpr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Duhamel KN, Valdimarsdottir HB, Bovbjerg DH. Psychological distress among healthy women with family histories of breast cancer: effects of recent life events. Psychooncology. 2005;14:555–563. doi: 10.1002/pon.870. [DOI] [PubMed] [Google Scholar]
- 8.Lerman C, Narod S, Schulman K, Hughes C, GomezCaminero A, Bonney G, et al. BRCA1 testing in families with hereditary breast-ovarian cancer—a prospective study of patient decision making and outcomes. J Am Med Assoc. 1996;275:1885–1892. [PubMed] [Google Scholar]
- 9.Esplen MJ, Hunter J, Leszcz M, Warner E, Narod S, Metcalfe K, et al. A multicenter study of supportive-expressive group therapy for women with BRCA1/BRCA2 mutations. Cancer. 2004;101:2327–2340. doi: 10.1002/cncr.20661. [DOI] [PubMed] [Google Scholar]
- 10.Meiser B. Psychological impact of genetic testing for cancer susceptibility: an update of the literature. Psychooncology. 2005;14:1060–1074. doi: 10.1002/pon.933. [DOI] [PubMed] [Google Scholar]
- 11.Smith AW, Dougall AL, Posluszny DM, Somers TJ, Rubinstein WS, Baum A. Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psychooncology. 2008;17:767–773. doi: 10.1002/pon.1291. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton R. Genetics: breast cancer as an exemplar. Nurs Clin North Am. 2009;44:327–338. doi: 10.1016/j.cnur.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerman C, Schwartz MD, Lin TH, Hughes C, Narod S, Lynch HT. The influence of psychological distress on use of genetic testing for cancer risk. J Consult Clin Psychol. 1997;65:414–420. doi: 10.1037//0022-006x.65.3.414. [DOI] [PubMed] [Google Scholar]
- 14.Baum A, Friedman AL, Zakowski SG. Stress and genetic testing for disease risk. Health Psychol. 1997;16:8–19. doi: 10.1037//0278-6133.16.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Watson M, Foster C, Eeles R, Eccles D, Ashley S, Davidson R, et al. Psychosocial impact of breast/ovarian (BRCA 1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Br J Cancer. 2004;91:1787–1794. doi: 10.1038/sj.bjc.6602207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobel M, Hamilton JG, Moyer A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol. 2009;28:510–518. doi: 10.1037/a0014778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakowski SG, Valdimarsdottir HB, Bovbjerg DH, Borgen P, Holland J, Kash K, et al. Predictors of intrusive thoughts and avoidance in women with family histories of breast cancer. Ann Behav Med. 1997;19:362–369. doi: 10.1007/BF02895155. [DOI] [PubMed] [Google Scholar]
- 18.Luecken LJ. Childhood attachment and loss experiences affect adult cardiovascular and cortisol function. Psychosom Med. 1998;60:765–772. doi: 10.1097/00006842-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Krause N. Early parental loss and personal control in later life. J Gerontol. 1993;48(3):P117–P126. doi: 10.1093/geronj/48.3.p117. [DOI] [PubMed] [Google Scholar]
- 20.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoke LA. Psychosocial adjustment in children of mothers with breast cancer. Psychooncology. 2001;10:361–369. doi: 10.1002/pon.515. [DOI] [PubMed] [Google Scholar]
- 22.Osborn T. The psychosocial impact of parental cancer on children and adolescents: a systematic review. Psychooncology. 2007;16:101–126. doi: 10.1002/pon.1113. [DOI] [PubMed] [Google Scholar]
- 23.Stroebe M, Schut H, Stroebe W. Health outcomes of bereavement. Lancet. 2007;370:1960–1973. doi: 10.1016/S0140-6736(07)61816-9. [DOI] [PubMed] [Google Scholar]
- 24.Henderson BJ, Tyndel S, Brain K, Clements A, Bankhead C, Austoker J, et al. Factors associated with breast cancer-specific distress in younger women participating in a family history mammography screening program. Psychooncology. 2008;17:74–82. doi: 10.1002/pon.1201. [DOI] [PubMed] [Google Scholar]
- 25.Visser A, Huizinga GA, van der Graaf WTA, Hoekstra HJ, Hoekstra-Weebers JE. The impact of parental cancer on children and the family: a review of the literature. Cancer Treat Rev. 2004;30:683–694. doi: 10.1016/j.ctrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Kersting A, Kroker K. Prolonged grief as a distinct disorder, specifically affecting female health. Arch Women. Ment Health. 2010;13:27–28. doi: 10.1007/s00737-009-0112-3. [DOI] [PubMed] [Google Scholar]
- 27.Luecken LJ, Appelhans BM. Early parental loss and salivary cortisol in young adulthood: the moderating role of family environment. Dev Psychopathol. 2006;18:295–308. doi: 10.1017/S0954579406060160. [DOI] [PubMed] [Google Scholar]
- 28.Krause N. Early parental loss, recent life events, and changes in health among older adults. J Aging Health. 1998;10:395–421. doi: 10.1177/089826439801000401. [DOI] [PubMed] [Google Scholar]
- 29.Nicolson NA. Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology. 2004;29:1012–1018. doi: 10.1016/j.psyneuen.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Lachman ME, Maier EH. Consequences of early parental loss and separation for health and well-being in midlife. Int J Behav Dev. 2000;24:183–189. [Google Scholar]
- 31.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer-Therapy (FACT) Scale. Cancer. 1995;75:1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Williamson G. Perceived Stress in a Probability Sample in the United States. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 33.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Burnett P, Middleton W, Raphael B, Martinek N. Measuring core bereavement phenomena. Psychol Med. 1997;27:49–57. doi: 10.1017/s0033291796004151. [DOI] [PubMed] [Google Scholar]
- 35.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 36.Manabe I. Chronic inflammation links cardiovascular, metabolic, and renal diseases. Circ J Off J Jpn Circ Soc. 2011;75:2739–2748. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- 37.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine, and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Känel R, Bellingrath S, Kudielka BM. Association between burnout and circulating levels of pro- and anti-inflammatory cytokines in schoolteachers. J Psychosom Res. 2008;65:51–59. doi: 10.1016/j.jpsychores.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 40.Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol. 2006;57(Suppl 11):5–29. [PubMed] [Google Scholar]
- 41.Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 42.Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-α receptor subtypes is essential for optimal skin tumor development. Oncogene. 2004;23:1902–1910. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- 43.Karabela SP, Kairi CA, Magkouta S, Psallidas I, Moschos C, Stathopoulos I, et al. Neutralization of tumor necrosis factor bioactivity ameliorates urethane-induced pulmonary oncogenesis in mice. Neoplasia. 2011;13:1143–1151. doi: 10.1593/neo.111224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defense and inflammatory diseases. Immunology. 2011;134:8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73:724–730. doi: 10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson EL, Wenzel LB, Osann K, Dogan-Ates A, Chantana N, Reina-Patton A, et al. Stress, immunity, and cervical cancer: biobehavioral outcomes of a randomized clinical trial [corrected] Clin Cancer Res. 2008;14:2111–2118. doi: 10.1158/1078-0432.CCR-07-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graves KD, Wenzel L, Schwartz MD, Luta G, Wileyto P, Narod S, et al. Randomized Controlled Trial of a Psychosocial Telephone Counseling Intervention in BRCA1 and BRCA2 mutation carriers. Cancer Epidem Biomar. 2010;19:648–654. doi: 10.1158/1055-9965.EPI-09-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]