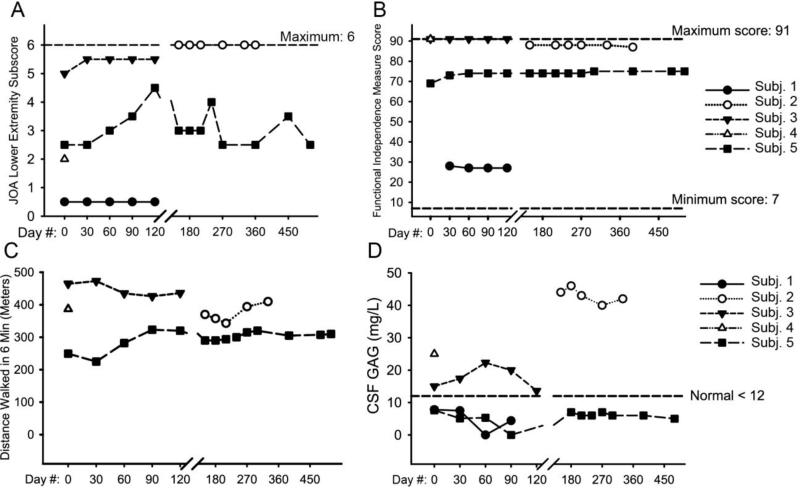
Fig. 1.
Efficacy outcomes in study subjects. (A) JOA score for the lower extremity. Intrasubject change (mean ± SD) during the 4-month pilot study was 0.83 ± 1.04, 95% CI (−1.75; 3.42), n = 3. (B) Functional independence measure in the subjects, which is a patient-reported measure of ability to perform activities of daily living. Intrasubject change (mean ± SD) during the 4-month pilot study was 1.33 ± 3.22, 95% CI (−6.65; 9.32), n = 3. (C) Six-minute walk test results. Subject 1 was nonambulatory and did not perform a six-minute walk test. Intrasubject change (mean ± SD) during the 4-month pilot study was 26.4 ± 56.0, 95% CI (−477; 530), n = 2. (D) CSF GAG measurements, which were taken immediately pre-dose at each dosing visit. Intrasubject change (mean ± SD) during the 4-month pilot study was 4.13 ± 3.16, 95% CI (−3.73; 12.0), n = 3. JOA: Japanese Orthopedic Association; CSF: cerebrospinal fluid; GAG: glycosaminoglycans; SD: standard deviation, CI: confidence interval.