Abstract
Five subjects with mucopolysaccharidosis type I and symptomatic cervical spinal stenosis received intrathecal laronidase in a 4-month pilot study and/or a 12-month extension study [1]. Clinical descriptions of study subjects, nonserious adverse events, individual data tables, and scoring system methods are provided. There were ten nonserious adverse events that occurred in more than one study subject. Somatosensory evoked potentials were absent in two subjects and normal in two subjects, limiting their utility as an endpoint. There were no significant changes in magnetic resonance imaging of cervical spinal cord or brain, pulmonary function tests, or cerebrospinal fluid opening pressure. These data are presented along with the scoring methods used in evaluation of the study subjects.
Specifications table
| Subject area | Biology |
| More specific subject area | Inborn errors of metabolism |
| Type of data | Text files, tables, figures |
| How data was acquired | Clinical evaluations, magnetic resonance imaging, nerve conduction studies, spirography, lumbar spinal puncture |
| Data format | Raw, processed |
| Experimental factors | Subjects were treated with intrathecal laronidase, and pre- and post-treatment data are presented |
| Experimental features | Clinical data are presented descriptively. Adverse events were tabulated based on subject reporting. Magnetic resonance imaging data were scored for spinal cord compression, hydrocephalus, white matter hyperintensities, and cystic or cribriform changes. Pulmonary function tests, cerebrospinal fluid opening pressure and somatosensory evoked potentials were obtained in standard clinical fashion. |
| Data source location | N/A (multicenter clinical trial) |
| Data accessibility | N/A |
Value of the data
-
•
There are very few clinical data reports of cervical spinal stenosis due to mucopolysaccharidosis type I, or attempts at treatment.
-
•
Quantitative as well as qualitative data are presented.
-
•
The data may inform future studies of intrathecal enzyme replacement therapy for lysosomal storage disorders.
1. Experimental design, materials and methods
1.1. Subjects
Study subjects are narratively described in data file 1. They were treated with intrathecal laronidase as described in Ref. [1].
1.2. Measures of safety
To evaluate possible adverse effects of study treatments, participants had physical and neurologic examination before and after each study treatment. All new physical complaints were evaluated and recorded including their severity and attribution to study treatments. Table 1 lists nonserious adverse events.
Table 1.
Nonserious adverse events.
| Events occurring in >1 study subject | Events occurring in 1 study subject |
|---|---|
| Headache | Anemia |
| Neck pain | Nausea |
| Hypoxemia | Pneumonia |
| Desaturation during procedure | Tingling |
| Elevated CSF opening pressure | Decreased sensation right hand |
| Back pain | Blurred vision |
| Myoclonic-like twitches | Decreased visual acuity |
| Diarrhea/gastroenteritis | Weakness |
| Upper respiratory infection | Insomnia |
| Fever | Elevated serum phosphorus |
| Decreased serum protein | |
| Decreased serum albumin | |
| Elevated CSF protein | |
| Elevated CSF leukocyte count | |
| Hip and thigh pain | |
| Pruritus (with fentanyl) | |
| Pneumocephaly | |
| Dizziness | |
| Gradual turning outward of right foot hampering walking and balance | |
| Abdominal pain | |
| Clumsiness in leg | |
| Dyspnea and cough | |
| Joint Pain | |
| Hand cramps | |
| Perineal muscle cramps | |
| Tachycardia at rest | |
| Sore throat | |
| “Shaking while laying down”/“tremor” | |
| Abdominal cramping / dysmenorrhea | |
| Facial flushing | |
| Urinary urgency | |
| Influenza | |
| Leg weakness | |
| Urticaria | |
| Melena | |
| Malaise |
CSF: cerebrospinal fluid.
1.3. Objective measures of efficacy
Response to treatment was assessed using a combination of subjective and objective measures. We evaluated somatosensory evoked potentials in the upper and lower extremity as per [2]. Results are shown in Table 2. MRI of brain and spinal cord were obtained to assess degree of cord compression and measurement of meningeal thickness was taken. MRI were performed using a 1.5-Tesla GE LX9.1. Brain imaging included sagittal T1-weighted, axial FLAIR, axial T2-weighted and axial diffusion-weighted images. Sagittal T1- and T2-weighted images of the whole spine and axial T1-weighted images of the cervical spine were obtained. Axial T1-weighted studies of the cervical spine were used to score spinal cord compression according to the methods of Houten and Cooper [3]. Brain images were evaluated for abnormal signal intensity in T2, enlargement of perivascular spaces, and ventricular size as per Matheus et al. [4]. Results are shown in Tables 3–7. Subjects enrolled in the extension study also underwent pulmonary function testing using spirometry (Fig. 1). Cerebrospinal fluid glycosaminoglycans were measured at Seattle Children׳s Hospital using a clinically-available test (Fig. 2). The laboratory uses a dimethylene blue dye-binding assay to quantitate total glycosaminoglycans [5]. Functional Independence Measure (FIM) score and Japanese Orthopedic Association (JOA) score measures were used to assess any changes in functional status and myelopathy. Results are shown in Fig. 1 of Ref. [1]. Scoring criteria for JOA and FIM are given in data files 2 and 3. The grading systems that were used to indicate the severity of spinal cord compression and brain imaging findings are given in data file 4.
Table 2.
Somatosensory evoked potentials. Intrasubject change (absolute) in latency (ms) during the pilot and extension studies for subjects with both baseline and end-study measurements.
|
4-Month pilot study |
12-Month extension study | |||
|---|---|---|---|---|
| Subject number: | 1 | 3 | 5 | 5 |
| Median nerve | ||||
| N9-N13A | Absent | 0.80 | Absent | Absent |
| N9-N13B | Absenta | −0.60 | Absent | Absent |
| N9-P13 | Absent | 1.10 | Absent | Absent |
| N13A-N20 | Absent | −0.40 | Absenta | 6.5 |
| Posterior tibial nerve | ||||
| N22-P30 | Absent | 1.00 | Absent | Absent |
| N22-P40 | Absent | 0.80 | 2.40 | 8.8 |
N9–N13B was present at baseline in subject 1 at a latency of 3.30 ms but absent at the Day 120 visit. N13A-N20 was present in subject 5 at the Day 120 visit at a latency of 10.8 ms but absent at baseline. Subject 2 did not have baseline measurements (the subject was not enrolled in the pilot study). Subject 4 did not have day 120 measurements (termination of participation due to subject death).
Table 3.
MRI cervical spinal cord compression (maximum grade).
|
4-Month pilot study |
12-Month extension study |
||||
|---|---|---|---|---|---|
| Subject number | Baseline | End of study | Baseline | Six months | End of study |
| 1 | 3 | 3 | 3 | N/A | N/A |
| 2 | N/A | N/A | 3 | 3 | N/A |
| 3 | 2 to 3 | 2 to 3 | N/A | N/A | N/A |
| 4 | 2 | N/A | N/A | N/A | N/A |
| 5 | 1 | ND | 1 | 2 | 2 |
Grading system: 0, 360° cushion of CSF around cord; 1, loss of CSF cushion w/o indentation of the cord but may have slight anterior cord flattening; 2, mild spinal cord compression; and 3, severe cord compression.
Table 4.
Brain MRI abnormal T2 signal intensity.
|
4-Month pilot study |
12-Month extension study |
||||
|---|---|---|---|---|---|
| Subject number | Baseline | End of Study | Baseline | Six months | End of study |
| 1 | 2 | 2 | 2 | N/A | N/A |
| 2 | N/A | N/A | 0 | 0 | N/A |
| 3 | 0 | 0 | N/A | N/A | N/A |
| 4 | 2 | N/A | N/A | N/A | N/A |
| 5 | 1 | 1 | 1 | 2 | 2 |
Grading system: score signal changes on T2-weighted images as 0, absent; 1, patchy and confined to the periventricular area; 2, patchy but in other white matter areas as well as periventricular; and 3, diffuse.
Table 5.
Brain MRI enlargement of perivascular spaces.
|
4-Month pilot study |
12-Month extension study |
||||
|---|---|---|---|---|---|
| Subject number | Baseline | End of study | Baseline | Six months | End of study |
| 1 | 2 | 2 | 3 | N/A | N/A |
| 2 | N/A | N/A | 1 | 1 | N/A |
| 3 | 0 | 0 | N/A | N/A | N/A |
| 4 | 2 | N/A | N/A | N/A | N/A |
| 5 | 3 | 2 | 3 | 2 | 2 |
Grading system: 0, no enlargement; 1, <3 mm enlargement; 2, between 3 and 8 mm enlargement; and 3, >8 mm enlargement.
Table 6.
Brain MRI frontal-occipital horn ratio.
|
4-Month pilot study |
12-Month extension study |
||||
|---|---|---|---|---|---|
| Subject number | Baseline | End of study | Baseline | Six months | End of study |
| 1 | 0.418 | 0.406 | 0.368 | N/A | N/A |
| 2 | N/A | N/A | 0.472 | 0.472 | N/A |
| 3 | 0.767 | 0.752 | N/A | N/A | N/A |
| 4 | 0.547 | N/A | N/A | N/A | N/A |
| 5 | 0.385 | 0.385 | 0.385 | 0.588 | 0.598 |
FOR is calculated as follows: measure a ratio between the frontal and occipital horns of the lateral ventricle using the maximum distance between the outer borders of the frontal horns added to the distance between the outer borders of occipital horns, then divide by twice the maximum biparietal diameter.
Table 7.
Brain MRI atrophy and megacisterna magna.
|
4-Month pilot study |
12-Month extension study |
||||
|---|---|---|---|---|---|
| Subject number | Baseline | End of study | Baseline | Six months | End of study |
| Brain atrophy | |||||
| 1 | 1 | 1 | 1 | N/A | N/A |
| 2 | N/A | N/A | 0 | 0 | N/A |
| 3 | 0 | 0 | N/A | N/A | N/A |
| 4 | 0 | N/A | N/A | N/A | N/A |
| 5 | 1 | 1 | 1 | 1 | 1 |
| Megacisterna magna | |||||
| 1 | 1 | 1 | 1 | N/A | N/A |
| 2 | N/A | N/A | 1 | 1 | N/A |
| 3 | 1 | 1 | N/A | N/A | N/A |
| 4 | 0 | N/A | N/A | N/A | N/A |
| 5 | 0 | 0 | 0 | 0 | 0 |
Grading system: 0, absent; 1, mild; 2, moderate; and 3, severe.
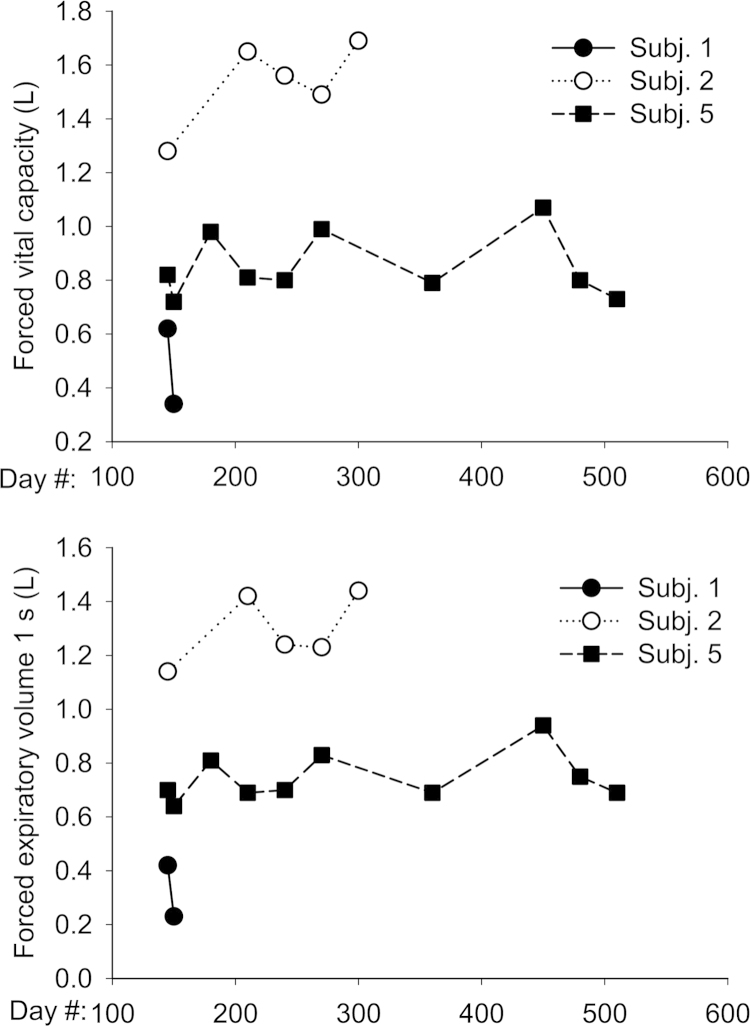
Fig. 1.
Pulmonary function tests. Subjects in the extension study underwent pulmonary function tests by spirometry at each visit.
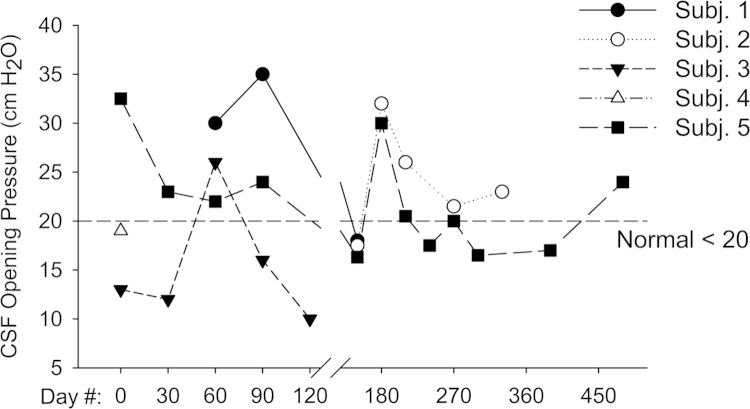
Fig. 2.
CSF opening pressure. We measured opening pressure prior to each dose of intrathecal laronidase by manometry at the level of the right atrium. We did not obtain opening pressure measurements at Days 0 and 30 for subject 1 due to technical issues performing the lumbar puncture during those visits. Subjects 2–4 had a history of hydrocephalus and implanted CSF drainage shunts.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2015.08.004.
Appendix A. Supporting information
Supplementary data
Supplementary data
Supplementary data
Supplementary data
References
- 1.Dickson P., Kaitila I., Harmatz P., Mlikotic A., Chen A., Victoroff A., Passage M., Madden J., Le S., Naylor D. Safety of laronidase delivered into the spinal canal for treatment of cervical stenosis in mucopolysaccharidosis I. Mol. Genet. Metab. 2015 doi: 10.1016/j.ymgme.2015.07.005. (PMID 26260077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boor R., Miebach E., Br++hl K., Beck M. Abnormal somatosensory evoked potentials indicate compressive cervical myelopathy in mucopolysaccharidoses. Neuropediatrics. 2000;31:122–127. doi: 10.1055/s-2000-7495. [DOI] [PubMed] [Google Scholar]
- 3.Houten J.K., Cooper P.R. Laminectomy and posterior cervical plating for multilevel cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: effects on cervical alignment, spinal cord compression, and neurological outcome. Neurosurgery. 2003;52:1081–1088. [PubMed] [Google Scholar]
- 4.Matheus M.G., Castillo M., Smith J.K., Armao D., Towle D., Muenzer J. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiology. 2004;46:666–672. doi: 10.1007/s00234-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 5.Whitley C.B., Draper K.A., Dutton C.M., Brown P.A., Severson S.L., France L.A. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin. Chem. 1989;35:2074–2081. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data