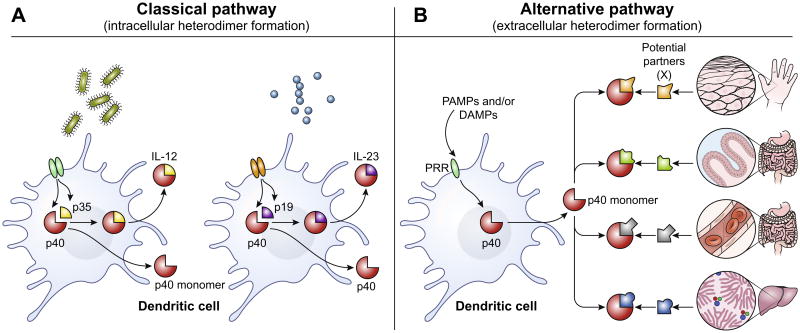
Fig. 1.
(A) The classical pathway of IL-12 and IL-23 secretion. First, IL-12p40 is induced in dendritic cells by inflammatory signals (PAMPs and/or DAMPs). Depending on the nature of the insult (e.g. type of pathogen, site of injury etc.), subsequent signals from NK, T or NKT cells (IFNγ, CD40L etc.,) can synergize to induce the expression of p35 or p19 on these DCs. These collective signals lead to the intracellular assembly of IL-12 (p40 plus p35) or IL-23 (p40 plus p19) and the secretion of these covalently linked heterodimers. (B) The alternative pathway. The induction of gene expression in this pathway is mechanistically identical to the classical pathway i.e. p40 is rapidly induced in response to PAMPs or DAMPS. However, the free p40 monomer secreted in the extracellular milieu will pair and bind with various potential partners available locally leading to the generation of new heterodimeric cytokines extracellularly (p40 plus X, where X is any one of many binding partners). The versatility of p40 monomer binding to various partners can be due to the nature of tissue in which immune response is being generated - e.g. skin epithelial cells may potentially have different binding partners for p40 monomer than those of intestinal villi, capillary endothelium or Liver hepatocytes. In addition (not shown) the specific pathogen or injury to the stromal tissues may also result in the release of different binding partners, and thereby influence the particular binding partner available at any given time. This allows p40 to form complexes tailored to each local context - and then convey that information by binding to IL-12Rβ containing receptors on other immune cells downstream of DC activation.