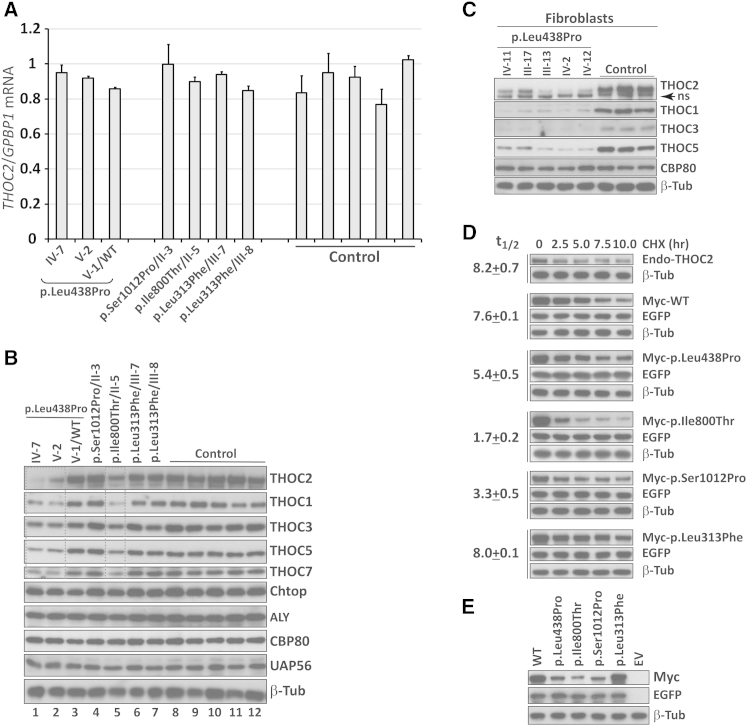
Figure 2.
Mutations Affect THOC2 Stability, Whereas mRNA Expression Is Unaltered
(A) THOC2 mRNA expression remained unaltered in LCLs derived from affected individuals in comparison to expression in LCLs from control individuals. Amounts of THOC2 mRNA were assayed by qRT-PCR and normalized to the housekeeping gene GPBP1’s mRNA. Primers are listed in Table S3. Error bars show SDs, which were calculated from three independent experiments.
(B) THOC2 (and THOC1, THOC3, THOC5, and THOC7) amounts were lower in p.Leu438Pro and p.Ile800Thr altered cells (boxed), but not in p.Ser1012Pro or p.Leu313Phe LCLs, than in control LCLs. Protein lysates from control and mutant LCLs were analyzed by western blotting. β-tubulin probing was used as a loading control. LCL proteins were extracted and western blotting was performed as reported previously.23 Western blots were probed with the following antibodies: rabbit anti-THOC2 (303-629A), rabbit anti-NCBP1/CBP80, rabbit anti-THOC1, rabbit anti-Aly, rabbit anti-SRAG/Chtop (Bethyl laboratories), rabbit anti-THOC7, rabbit anti-THOC3 (Sigma-Aldrich), rabbit anti-CIP29 (Thermo Scientific), and rabbit β-tubulin (Abcam).
(C) Amounts of p.Leu438Pro THOC2 were reduced in fibroblasts from five different affected individuals from the MRX12 family. Protein lysates from control and mutant fibroblasts were analyzed by western blotting with the antibodies shown. Probing for β-tubulin was used as a loading control.
(D) Mutations affect THOC2 turnover. For generating Myc-tagged human THOC2 expression plasmids, THOC2 coding sequence was PCR amplified from brain cDNA with hTHOC2-XbaI-XhoI-F and hTHOC2-NotI-R primers (Table S3) and cloned at XhoI-NotI sites in the pCMV-Myc mammalian expression vector. Each mutation was introduced by the overlap PCR method using the primers listed in Table S3. All constructs were validated by Sanger sequencing. Untransfected HEK293T cells or HEK293T cells transfected with wild-type or altered Myc-tagged THOC2 and EGFP expression plasmid were treated with the translation inhibitor cycloheximide (CHX) and harvested at different time points after CHX addition. Cell lysates were analyzed by western blotting with an antibody to the Myc tag, EGFP (transfection control), or β-tubulin (loading control). Whereas amounts of the endogenous, wild-type, or p.Leu313Phe THOC2 proteins declined slowly over a period of 8.0 hr, p.Leu438Pro (5.4 hr), p.Ile800Thr (1.7 hr), or p.Ser1012Pro (3.3 hr) amounts declined more rapidly. The amounts of THOC2 and loading-control β-tubulin were measured by quantification of the band intensities with ImageJ software. THOC2 amounts were relativized to a β-tubulin loading control for determining the half-life of proteins. Each experiment was repeated three times, and the results of one representative experiment are shown. Data shown are the mean ± SD. Note that western blot signals for p.Leu438Pro, p.Ile800Thr, and p.Ser1012Pro at 0 hr look different than those in Figure 2E. This is because blots were exposed for durations different to those shown in Figure 2E.
(E) THOC2 variants affect stability of ectopic recombinant Myc-tagged THOC2 proteins. Total lysates of HEK293T cells transfected with wild-type THOC2 (WT), altered Myc-tagged THOC2, or empty vector (EV) and EGFP expression plasmid were analyzed by western blotting with an antibody to the Myc tag, EGFP (transfection control), or β tubulin (loading control).