Figure 3. Clock-controlled NAD+ levels regulate enzymatic activity of SIRT1 and PARP1.
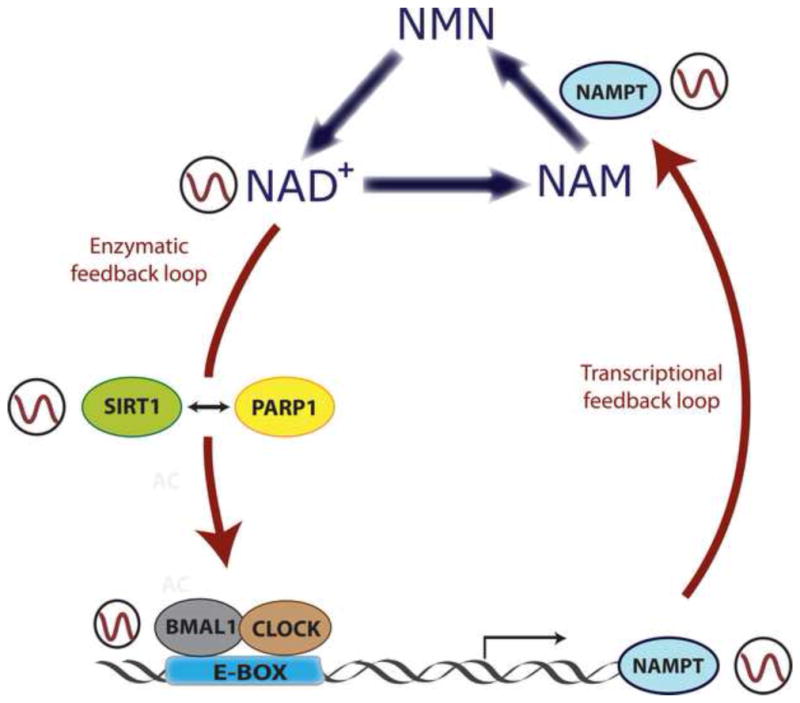
Intracellular levels of NAD+ oscillate in a circadian manner, and this oscillation is driven by the molecular clock. CLOCK:BMAL1 complexes rhythmically bind to E-boxes at the Nampt gene promoter, directing its circadian transcription. The resulting NAMPT protein is a key rate-limiting enzyme in the NAD+ salvage pathway, which dictates the circadian biosynthesis of NAD+. This metabolite feeds back into the clock by serving as a cofactor for SIRT1 and PARP1 enzymes, whose enzymatic activity is circadian. SIRT1 and PARP1 directly target CLOCK:BMAL1 complexes to change their transactivational activity. A molecular interplay between SIRT1 and PARP1 exists, and depends on NAD+ levels. By this mechanism, NAD+ intersects in between a transcriptional and an enzymatic feedback loop to regulate energy homeostasis.
