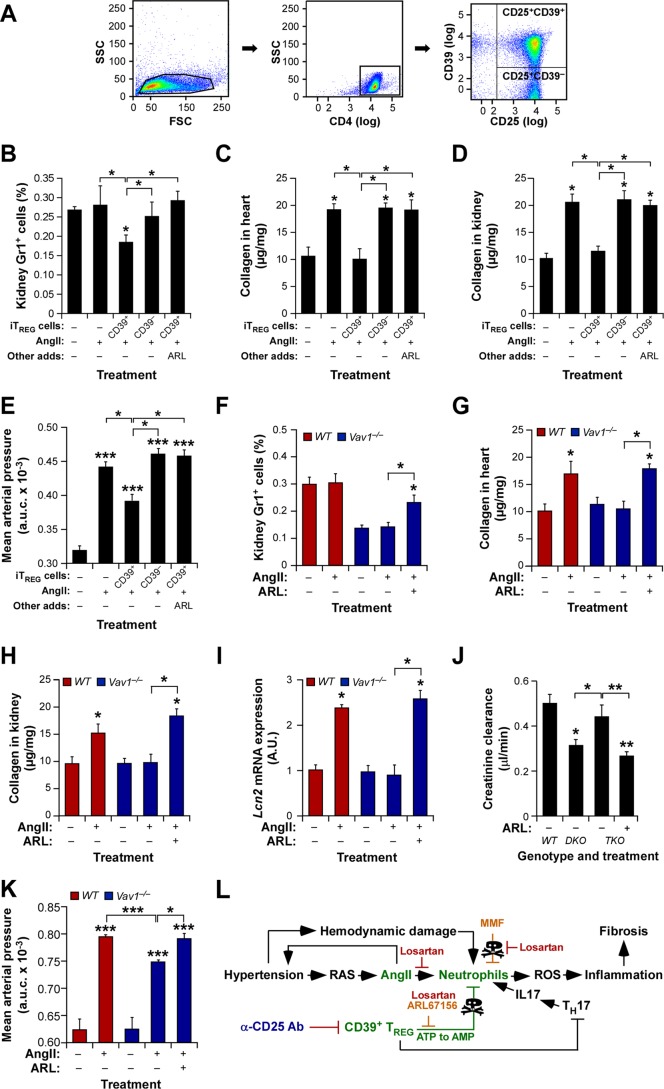
Fig 9.
CD39+ TREG cells are involved in the protection against AngII-driven cardiorenal dysfunctions. (A) Example of the flow cytometry-mediated purification of CD39+ and CD39− iTREG cells used in the experiments for which results are shown in panels B to E. (B to E) Percentages of kidney-infiltrating neutrophils (B), extent of cardiorenal fibrosis (C and D), and blood pressure levels (E) in WT mice under the indicated experimental conditions. Asterisks indicate significant differences (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001) from the control or between the indicated experimental groups (n = 4). (F to K) Percentages of kidney-infiltrating neutrophils (n = 4) (F), cardiorenal fibrosis levels (n = 4) (G and H), abundance of Lnc2 transcripts in kidneys (n = 4) (I), creatinine clearance rates (n = 6) (J), and overall blood pressure levels (n = 4) (K) in mice of the indicated genotypes under the indicated experimental conditions. Asterisks indicate significant differences (*, P ≤ 0.05; **, P ≤ 0.05; ***, P ≤ 0.001) from the control or between the indicated experimental groups. (L) The new mechanism (green) described in this work. Inhibitors tested exclusively in vitro or in vivo are shown in red or blue letters, respectively. Those used under both conditions are shown in light brown letters. The TREG-TH-neutrophil connection is proposed on the basis of previously published data (1, 2, 7, 8, 22). RAS, renin-angiotensin system.