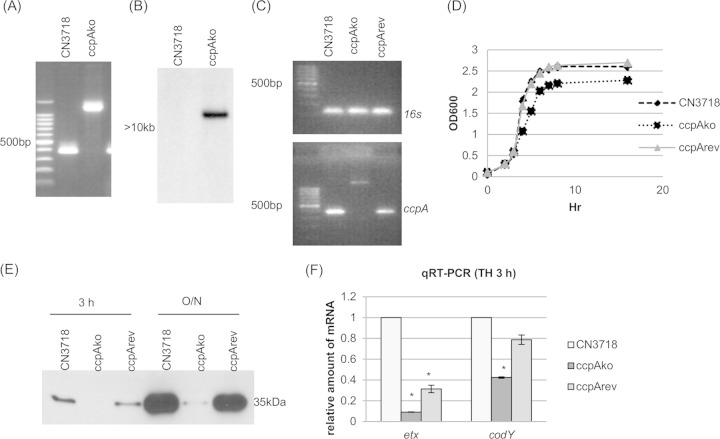
FIG 6.
ccpA null mutant strain preparation and characterization. (A) PCR confirmation of the construction of an isogenic ccpA null mutant strain. Using DNA isolated from wild-type strain CN3718, a PCR using internal ccpA gene primers amplified the expected product of about 400 bp. Using DNA template isolated from the ccpAko strain, which has a 900-bp intron insertion in the ccpA gene, the same PCR assay amplified a larger product of 1,300 bp. The leftmost, unlabeled lane contains a 100-bp molecular ruler. (B) Intron-specific Southern blot hybridization with DNA from wild-type or ccpAko strain. DNA from each strain was digested with EcoRI and electrophoresed on a 1% agarose gel. The size of the DNA fragment is indicated in kilobases. (C) RT-PCR analysis for ccpA transcription of CN3718, ccpAko, or ccpArev strain. (Top) Transcription of housekeeping gene 16S RNA gene; (bottom) transcription of ccpA gene. The leftmost, unlabeled lane contains a 100-bp molecular ruler. (D) Postinoculation change in optical density (OD600) for cultures of wild-type CN3718, the ccpA null mutant strain ccpAko, and reversed mutant strain ccpArev growing in TH medium at 30°C. (E) ETX Western blot analysis of CN3718, ccpAko, and ccpArev strains grown in TH medium culture at 30°C for 3 h or overnight (O/N). (F) Quantitative RT-PCR analyses of etx or codY transcription for a 3-h TH culture of CN3718, ccpAko, or ccpArev strain. Average CT values were normalized to that of the housekeeping 16S RNA gene, and the fold differences were calculated using the comparative CT method (2−ΔΔCT). Each bar indicates the calculated fold change relative to wild-type CN3718. All experiments were repeated three times, and mean values are shown. The error bars indicate standard deviations. *, P < 0.005 compared to wild-type strain by ordinary one-way ANOVA.