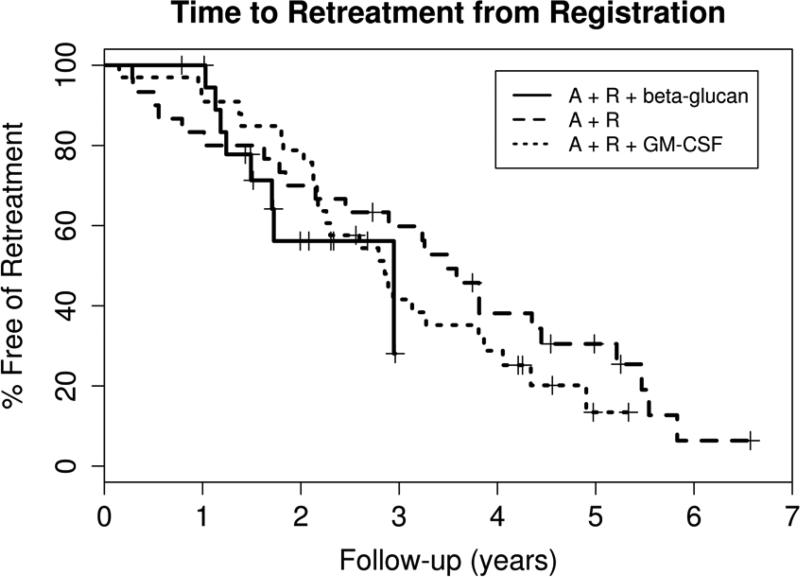
Figure 2. Time to Retreatment.
The Kaplan-Meier survival curves for time to treatment from three sequential phase II clinical trials using the same 5-week regimen of alemtuzumab and rituximab for early treatment of high risk CLL are plotted for comparison. MCO38G [3] used only alemtuzumab and rituximab (A+R) and enrolled 30 patients with a median time of follow up of 5.2 years (range 2.7–8.2). Twenty-four (80%) patients required retreatment for progressive CLL at a median time of 3.5 years (95% CI 2.1, 4.4). The subsequent trial MC0785 [4] added GM-CSF to the regimen (A+R+GM-CSF) and enrolled 33 patients with a median follow up of 5.0 years (range 3.9-5.6). Twenty-six (79%) of these patients have required retreatment at a median time of 2.9 years (95% CI 2.2, 3.8). The LS1084 study reported in this paper (A+R+ beta-glucan) enrolled 20 eligible patients with a median follow up of 2.0 years (range 0.8 – 3.1). Eight (40%) of these patients have required retreatment with a median time to retreatment of 2.9 years (range 1.5 to not reached).