ABSTRACT
Evaluation of 30 cases of craniopharyngioma treated by Gamma Knife at Nagoya Radiosurgery Center (NRC), Nagoya Kyoritsu Hospital since July, 2004 has been made. The mean volume of the tumor was 2.64 ml, which was treated with the marginal dose of 11.7 Gy. Mean follow-up period was 79.9 months. The effects were evaluated by MRI findings, neuro-endocrine and hypothalamic signs and symptoms, complications and KPS every 3~6 months. As the results, complete remission was obtained in 8, partial remission in 12, no change in 6, progression in 3, in which two died by hypothalamic invasion. Tumor response rate was 68.9% and control rate 87.9%. Actuarial survival was 96% at 5 and 86% at 10 years. However, progression free survival was 76% and 76%, respectively. Using marginal dose of 11.7Gy to a smaller tumor, better control without complications has been obtained. KPS was excellent in 14, good in 9, fair in 2, poor in 1 and unknown in a case. Finally, there were three deaths, where two were died of tumor progression and one by infirmity. The progression of hypothalamic symptoms other than diabetes insipidus were found in two cases. Volume reduction and effective dose setting will be important for the improvement of QOL and survival after combined microsurgery and radiosurgery of craniopharyngioma.
Key Words: craniopharyngioma, gamma knife radiosurgery, tumor recurrence, hypothalamic involvement
INTRODUCTION
As craniopharyngioma is a congenital brain tumor and benign in nature, total surgical removal is the ideal treatment.1, 2) However, total removal is often difficult without deterioration of QOL of the patients because the tumor originates in the hypothalamic-pituitary axis. As the second choice, the residual tumor after surgery or the recurrent tumor had been treated by fractionated-radiotherapy, adjuvantly or additionally.3-5) However, not only complications to optic nerves, pituitary gland and hypothalamus, but also control of the tumor has not been satisfied.4, 5) Recent progress of stereotactic irradiation such as gamma knife radiosurgery (GKR)10, 14-20) and stereotactic radiotherapy (SRT)11-13) have become accurate, and stereotactic treatments are now good indication for craniopharyngioma. One hundred and twenty eight cases of residual or recurrent craniopharyngioma have been successively treated by GKR & surgery since May 1991 at Komaki City Hospital.7) In this paper, recent 30 cases treated at Nagoya Kyoritsu Hospital during 10 years since July 2004 have been studied and discussed by comparison with those of previous papers.6, 7)
MATERIALS AND METHODS
Characteristics of 30 patients are shown. The mean age was 44.7 (6–86) years old. Male to female ratio was 19:11, Adult to child ratio was 26:4, The ratio of post surgical residual to recurrent tumor was 23:7. The location of tumors were suprasellar and/or intraventricular in 18, intrasellar in 9, and chiasm & anterior part in 3. The nature of tumors was solid in 11, cystic in 7, and mixed in 12. Mean size of tumors was 2.64 (0.3–9.3) ml in volume and 16.3 (8.31–23.5) mm in diameter. The prescribed dose of GKR was mean maximum of 23.6 (20–28) Gy and mean marginal of 11.7 (8.2–23) Gy. The follow-up period was 6 months to 10 years after GKR, in which mean of 79.9 and median of 91 months were obtained. As the previous surgical treatments, intracranial and transshpenoidal removals of tumor were made 37 and 13 times, respectively. V-P shunt in two and Ommaya reservoir setting in three cases were performed.
The evaluation of the effects was made by repeated MRIs, neuro-endocrine changes, hypothalamic signs & symptoms, complications, and QOL assessed by KPS every 3 to 6 months.
RESULTS
Twenty-nine out of 30 patients were evaluable because one case was lost to follow-up. The change of tumors was complete remission (CR) in 8, partial remission(PR) in 12, no change (NC) in 6 and progression(PG) in 3 patients, in which 2 died by progression of hypothalamic involvement and one by infirmity. The tumor response rate was 68.9% (20/29) and the control rate was 89.7 % (26/29). Regarding signs and symptoms, ten cases showed visual disturbance, in which 3 of visual acuity, 2 of the visual field and 5 of both disturbances were found. Sixteen cases showed endocrine disturbances with panhypopituitarism in 10, GH deficiency in 4, hypo-corticoid, hypo-gonad, hypo-TSH and hypo-IGF-1 in 1 before GKR. After GKR, there was no new appearance nor progression of neuro-endocrinological symptoms, and even an improvement of visual disturbance was found in 2 cases. There were 5 cases with hypothalamic signs and symptoms in which DI was found in all but other conditions were found in 3 cases such as electrolyte abnormality in 1, temperature disturbance in 1, appetite abnormality and obesity in 2 and hypersomnia in 2 cases. Hypothalamic signs and symptoms progressed in 2 cases, from which the patients died at 8.5 and 14 years after the first treatment. The outcome of patients was assessed by the doctors’ reports of KPS levels. The scores were excellent (KPS=100) in 14, good (90) in 9, fair (70–80) in 2 and poor (50–60) in a case and unknown in another.
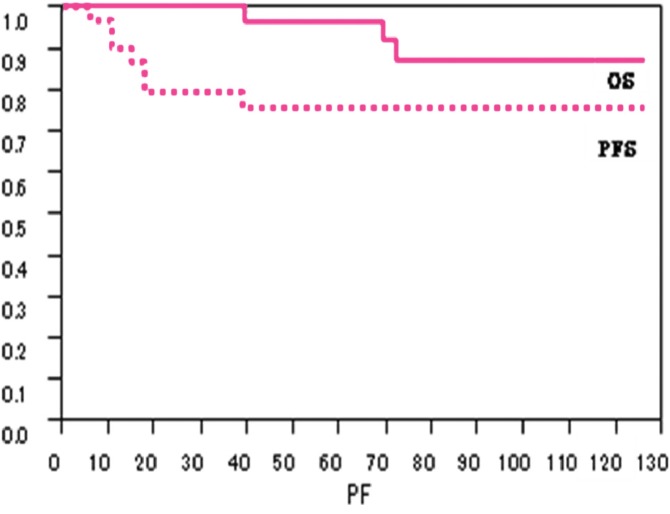
Actuarial survival by Kaplan-Meier method revealed 96% at 5 years and 86% at 10 years. PFS was 76% at 5 years and 76% at 10 years, respectively (Figure 1).
Fig. 1.
Survivals after Surgery and Gamma Knife Radiosurgery
OS= over all survival, PFS= progression free survival
Two patients presented unusual course with the hypothalamic invasion are shown.
Case 1. A 6-year-old boy showed visual disturbance and hydrocephalus in September, 1996. Before visiting NRC at Nagoya Kyoritsu Hospital, he had two craniotomies for subtotal removal of the tumor, and subsequent GKR in 1996 and 1999, where the marginal doses were 11 Gy and 11.5 Gy, respectively. The third craniotomy was made in December 2004 because the suprasellar tumor recurred, and was followed by a third GKR with the marginal dose of 10.5 Gy. Fourth surgical removal was made by transsphenoidal surgery for the recurrent intrasellar tumor, followed by a 4th GKR using the marginal dose of 14 Gy. Thereafter, bilateral visual field defects and a tendency of obesity appeared and progressed. In June 2006, obesity (body mass index; BMI>30%) and poikilothermia developed. After the tumor recurrences, three surgical removals and a 5th GKR was made using the marginal dose of 10.5 Gy. Daytime hypersomnia and attacks of loss of consciousness occurred from June 2009. The tumor became huge even after chemotherapy using Bleomycine (Figure 2). The patient died in August 2010 at the age of 19. In total, the patient had nine surgeries, five GKRs and one course of chemotherapy during the 14 years from diagnosis.
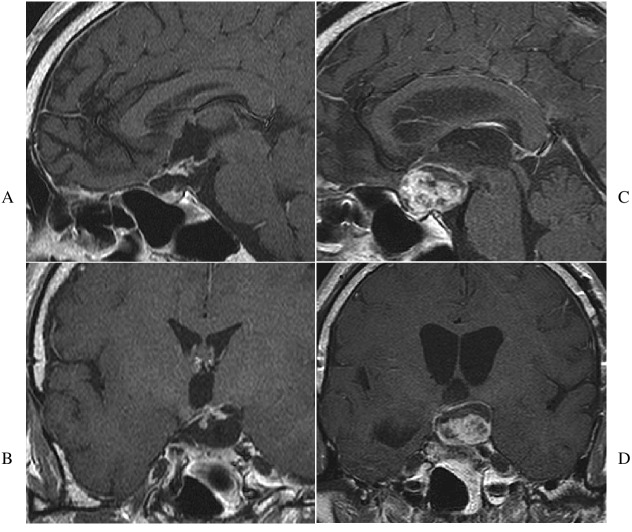
Fig. 2.
MRI Findings of Hypothalamic Involvement of Case 1
A: Mid-sagittal MRI showes supra- and intrasellar tumor invading the floor of anterior IIIrd ventricle, where patient showed obesity and poikilothermia.
B: Aggressive progression of the tumor into IIIrd ventricle and skull base is found after multiple surgeries, GKR and chemotherapy.
Case 2. A 42-year-old man complained of visual disturbance of the right eye and was diagnosed as suprasellar tumor in June 1999. Partial removal was made by craniotomy in October. The tumor regrew and a second craniotomy was made, followed by 50 Gy of focal fractionated radiotherapy. A cystic tumor recurred in September 2003 which was evacuated by the transsphenoidal approach. Third craniotomy was made for the setting of an Ommaya reservoir in December. After cyst evacuation, GKR was made to the tumor of 6.4 ml with a marginal dose of 9.5 Gy in March 2005 (Figure 3A). His visual acuity became "finger movement" and his right visual field hemianopsic. The tumor enlarged after intratumoral bleeding and patient showed hydrocephalus in October 2006 (Figure 3B). Although a VP shunt was made, the patient became disoriented and hypersomnic from February 2007. The patient showed attacks of loss of consciousness, high fever, and electrolyte imbalance since July. Because his family rejected further treatment, the patient died in February 2008.
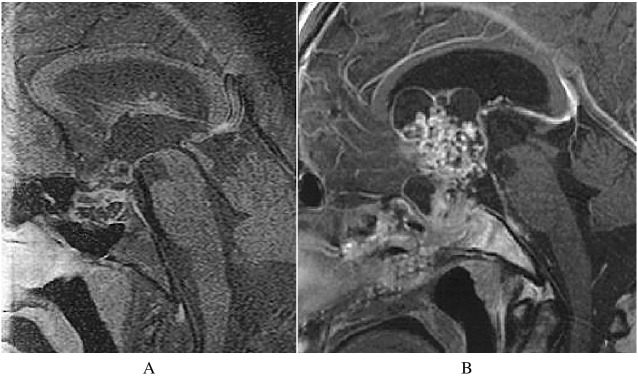
Fig. 3.
Sagittal and Coronal MRI Findings of Hypothalamic Involement in Case 2
Recurrent cystic tumor is found under anterior IIIrd ventricle after surgeries (A,B). After fractionated radiotherapy, Ommaya setting and GKR, the patient showed DI and visual disturbances. The tumor enlarged with hydrocephalus (C,D). Patient showed hypothalamic signs and symptoms such as hypersomnia, high fever and electrolyte disturbances, and died within seven months.
DISCUSSION
One of the important factor which makes the prognosis of craniopharyngioma worsen will be the regrowth or recurrence of tumors after treatments. Many prognostic factors have been documented for the recurrence,6- 7,10, 14-19) but none of them had been verified as significant factor. Recently, we have statistically analyzed nine factors which appeared in the past using our database.20) They are age, sex, child or adult, residual or recurrent tumor, natures of tumor, size of tumor, pathological diagnosis, numbers of previous treatments and radiation dose. By univariate and multivariate analysis, tumor size and radiation dose are the significant factors for recurrence of tumor after partial removal followed by GKR.8-10) Furthermore, ROC analysis* revealed that a mean diameter less than 19 mm and a marginal dose more than 13.2 Gy are favorable prognostic factors.20) Regarding two factors, results of present study are compaired with those of previous studies6, 7) (Table 1). Initial study presented 33 cases, in which a tumor volume of 4.4 ml was treated with the marginal dose of 12.8 Gy.6) The tumor control rate was 84.8%, but the optico-endocrine complications occurred relatively high as 15.2% (5 in 33 cases). While the next report with 98 cases that the tumor volume of 3.5 ml was treated with a marginal dose of 11.5 Gy revealed an actuarial survival of 94 % and 91% in 5 and 10 years, but PFS were 60% and 53%, respectively.7) However, the rate of neuro-endocrine complications decreased to 6.5% (6 in 98 cases). Finally, the present study of 30 cases in which the tumor volume of 2.6 ml was treated with a marginal dose of 11.7 Gy revealed a higher survival and PFS for 5 and 10 years without complications. Regarding the effective dose for craniopharyngioma, it was suggested that the dose should be higher than 11.5 Gy but less than 12.8 Gy and the marginal dose of 11.7Gy showed higher response and control rates. Massive hypothalamic invasion4, 19) was found in two of 30 patients (6.6%) in this study in which the incidence was similar to that of previous study.7) The tumor was originally cystic in Case 2 and mixed type in Case 1 which initially located at the anterior floor of third ventricle (Figure 2A, 3A), and grew huge to occupy whole hypothalamus until patients’ death at 8.5 and 14 years. It was again suggested that the dose of each treatment seemed too low to control tumor because of OARs, especially optic nerve nearby. However, the control of tumor and radiation injury to OAR are the both sides. And, recent developement of stereotactic irradiation technology for these tumors has become tumor control maximum but of radiation injury minimum.8, 9, 21,24)
Table 1.
Comparison of Results with Present and Previous Studies
| FU Studies, Year # of Cases (FU time) |
Tumor size (ml, mm) |
Marginal Dose (Gy) |
Survival Rate (%) |
PFS (M) |
Opticoendocrine Complications (%)* |
|---|---|---|---|---|---|
| 1) First Study, 1999 | |||||
| Radiosurgery | 4.4 20.3 | 12.8 | – | 84.8** | 5/31=15.3 |
| 33 Cases (42.1M) | |||||
| 2) Second Study, 2005 | |||||
| J Neurosurg. | 3.5 18.8 | 11.5 | 5Y=94 | 60 | 6/98= 6 |
| 98 Cases (65.5M) | 10Y=91 | 53 | |||
| 3) Third Study, 2014 | |||||
| (Present study) | 2.6 16.3 | 11.7 | 5Y=96 | 76 | 0/27=0 |
| 30 Cases (79.9M) | 10Y=86 | 76 | (improved 2/27) |
*Complication rate of the group 1) to 3) and group 2) to 3) are statistically significant (p<0.0371) by Fischer exact test.
**indicates percentage of mean survival time (M) of this group
Abbreviations: PPS=progression free survival, M=months, Y=years, FU=follow-up, Gy=grey
Chiou et al.15) had treated tumors using higher marginal dose of 16.4 Gy. Control and disappearance of tumor showed 80% and 40% respectively, however cyst enlargement in 30% and visual disturbance in 20% were found as complications.
While Ulfarsson et al.18) had used a lowest marginal dose of 5 Gy to mean volume of 8.0 ml, lower tumor control of 36% and response rate of 23% were obtained, along with a high recurrence rate of 63% and optic nerve injury of 42.1%.
Present study has confirmed that radiation injury was not found by applying relatively smaller marginal dose of 11.7 Gy to a small tumor volume of 2.6 ml. Also actuarial survivals were 96% and 86% for 5 and 10 years, and the PFSs at same times were 76%, respectively. This implies that the effective marginal dose could be around 11.7Gy to control the tumor without radiation injury. However, surgical reduction of tumor volume is another important issue to increase the effective dose as shown by present study and others.15-17, 19) Although so-called an optimal or effective dose for craniopharyngioma has not been defined, the marginal dose could be increased safely when a tumor is small. Both of present authors (T.K and Y.M) had successively engaged in GKR of craniopharyngioma since 19916) and treated consecutive 128 cases at two GK sites using common strategy of "volume reduction and setting of effective dose". Recently, many authors of GKR for craniopharyngioma have advocated the similar strategy to treat smaller tumor with higher radiation dose.14- 15,17, 19) It has recently been proposed by European investigators22-24) that for the treatment of pediatric craniopharyngiomas, the initial treatment should be less invasive and conservative to prevent crucial damages to the developing brain. In case of larger tumors or inoperable conditions, stereotactic fractionated radiotherapy11-13) has been recommended to prevent radiation injuries to the OAR after pathological diagnosis.
CONCLUSION
To improve the survival and QOL of patients with craniopharyngioma, residual or recurrent tumors should be treated by stereotactic irradiation using an effective dose to alleviate regrowth. The prognostic factors regarding recurrence were found to be radiation dose and tumor volume. The effective marginal dose will be around 11.7 Gy when the tumor size is small (2.64ml in volume or 16.3mm in diameter).
The appearance and progression of hypothalamic symptoms other than diabetes insipidus will be signs of poor prognosis, implying hypothalamic invasion of the tumor. Early diagnosis and establishment of effective treatments are crucial.
*ROC: a receiver operating characteristic (ROC) from Wikipedia, is a graphical plot of the sensitivity, or true positive rate, vs false positive rate
ACKNOWLEDGEMNT
This paper was presented at the 21th Annual Meeting of The Japanese Society for Stereotactic Radiosurgery at Maebashi on June 1st 2012 and the 4th Annual Meeting of The Japan Radiosurgery Society on January 19th, 2013.
CONFLICTS OF INTEREST DISCLOSURE
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices in this article.
REFERENCES
- 1).Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SL. Aggressive surgical management of craniopharyngioma in children. J Neurosurg, 1992; 76: 47–52. [DOI] [PubMed]
- 2).Yasargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas; Approarches and long-term results in 144 patients. J Neurosurg, 1990; 73: 3–11. [DOI] [PubMed]
- 3).Moon SH, Kim IH, Park SW, Kim I, Hong S, Park CL Wang KC, Cho BK. Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngioma -a study in single institute- Childs Nerv Syst, 2005; 21: 799–807. [DOI] [PubMed]
- 4).Pemberton LS, Dougal M, Magee B, Gattamaneni HR. Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol, 2005; 77: 99–104. [DOI] [PubMed]
- 5).Varlotto JM, Flickinger JC, Kondziolka D, Lunsford LD, Deutsch M. External beam irradiation of craniopharyngiomas; long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys, 2002; 54: 492–499. [DOI] [PubMed]
- 6).Kobayashi T, Kida Y, Mori Y. Effects and prognostic factors in the treatment of craniopharyngioma by gamma knife radiosurgery. Radiosurgery, 1994; vol 3: pp192–204.
- 7).Kobayashi T, Kida Y, Mori Y, Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg (6 Suppl Pediatr), 2005; 103: 482–488. [DOI] [PubMed]
- 8).Larson DA, Flickinger JC, Loeffler JS. The radiobiology of radiosurgery. Int J Radiat Oncol Biol Phys, 1933 25: 557–561. [DOI] [PubMed]
- 9).Marks LB, Spencer DP. The influence of volume on the tolerance of the brain to radiosurgery. J Neurosurg, 1991; 75: 177–180. [DOI] [PubMed]
- 10).Yomo S, Hayashi M, Chernov M, Tamura N, Izawa M, Okada Y, Hori T, Iseki H. Stereotactic radiosurgery of residual or recurrent caraiopharyngioma; new treatment concept using Leksell gamma knife model C with automatic positioning system. Stereotact Funct Neurosurg, 2009; 87: 360–367. [DOI] [PubMed]
- 11).Hashizume C, Mori Y, Kobayashi T, Shibamoto Y, Nagai A, Hayashi N. Stereotactic radiotherapy using Novalis for craniopharyngioma adjacent to optic pathways. J Neurooncol, 2010; 98: 239–247. [DOI] [PubMed]
- 12).Minniti G, Esposito V, Amichetti M, Enrici RM. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev., 2009; 32: 125–132. [DOI] [PubMed]
- 13).Selch MT, De Salles AA, Wade M, Lee SP, Solberg TD, Wallace RE, Ford JM, Rubino G, Gabatan-Awang C, Withers HR. Initial clinical results of stereotactic radiotherapy for the treatment of craniopharyngioma. Technol Cancer Res Treat, 2002; 1: 51–59. [DOI] [PubMed]
- 14).Amendola BE, Wolf A, Coy SR, Amendola MA. Role of Radiosurgery in craniopharyngioma; a preliminary report. Med Pediatr Oncol, 2003; 41: 123–127. [DOI] [PubMed]
- 15).Chiou SM, Lunsford LD, Niranjan A, Kondziolka D, Flickinger JC. Stereotactic radiosurgery of residual or recurrent craniopharyngioma, after surgery, with or without radiation therapy. Neurooncol, 2001; 3: 159–166. [DOI] [PMC free article] [PubMed]
- 16).Chung WY, Pan DHC, Shiau CY, Guo:WY, Wang LW. Gamma knife radiosurgery for craniopharyngioma. J Neurosurg, 2000; 93 (Suppl 3): 47–56. [DOI] [PubMed]
- 17).Niranjan A, Kano A, Mathieu D, Kondziolka D, Flickinger JC, Lunsford LD. Radiosurgery for craniopharyngioma. Int J Radiat Oncol Biol Phys, 2010; 78: 64–71. [DOI] [PubMed]
- 18).Ulfarsson E, Lindquist C, Roberts M, Rahn T, Lindquist M, Thoren M, Lippitz B. Gamma knife radiosurgery for craniopharyngioms : long–term results in the first Swedish patients. J Neurosurg (Suppl 5), 2002; 97: 613–622. [DOI] [PubMed]
- 19).Xu Z, Yen CP, Schlesinger D, Sheehan,J. Outcome of Gamma Knife radiosurgery for craniopharyngioma. J Neurooncol, 2011; 104: 305–313. [DOI] [PubMed]
- 20).Kobayashi T, Mori Y, Tsugawa T, Hashizume C, Takahashi H. Prognostic factors for tumor recurrence after gamma knife radiosurgery of partially resected and recurrent craniopharyngioma. Nagoya J Med Sci, 2012; 74: 141–147. [PMC free article] [PubMed]
- 21).Leber KA, Bergloff J, Pendle G. Dose-response tolerance of the visual pathways and caranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg, 1998; 88: 43–50. [DOI] [PubMed]
- 22).Gleeson H, Amin R, Maghnie M. ‘Do no harm’ management of craniopharyngioma. Eur J Endocr, 2006; 159: S95–S99. [DOI] [PubMed]
- 23).Muller HL, Bruhnken G, Emser A, Faldum:A, Etavard-Gorris N, Gebhardt U, Kolb R, Sorensen N. Longitudinal study on quality of life in 102 survivors of childhood craniopharyngioma. Childs Nerv Syst, 2005; 21: 975–980. [DOI] [PubMed]
- 24).Spoudeas HA, Saran F, Pizer B. A multimodarlity approach to the treatment of craniopharyngioma avoiding hypothalamic morbidity; A UK perspective. J Ped Endocr & Metab, 2006; 19: 447–451. [DOI] [PubMed]