Abstract
Introduction
Enhanced recovery is a concept that has become increasingly popular for arthroplasty surgery over the last ten years. This study was designed to assess the analgesia requirements, pain levels and time to discharge for patients having primary arthroplasty in the enhanced recovery pathway.
Methods
A multidisciplinary prospective cohort study was carried out between January 2012 and March 2012. Data were collected for patients undergoing primary arthroplasty in one hospital during this time. Details of anaesthesia, local infiltration, additional medications and analgesia were recorded. A visual analogue scale pain score was obtained from each patient at time of mobilisation on days 0, 1, 2 and 3 postoperatively.
Results
Ninety-six patients were included in the study. Of these, 34 underwent total hip arthroplasty and 62 total knee arthroplasty (TKA). Pain was the greatest contributor for delayed discharge in TKA patients. The patients who had TKA and did not receive non-steroidal anti-inflammatory drugs (NSAIDs) had significantly higher pain scores (day 0, p<0.01; day 1, p<0.001; day 2, p<0.01) and significantly increased opiate demands compared with those patients who did receive NSAIDs.
Conclusions
There are unacceptably high pain scores in patients undergoing TKA without the use of NSAIDs. There should be focused intervention with this group of patients to improve their pain scores and reduce their length of stay.
Keywords: Enhanced recovery, Fast track orthopaedics, Postoperative arthroplasty pain, Non-steroidal anti-inflammatory drugs
Enhanced recovery is a concept that has become increasingly popular for arthroplasty surgery over the last ten years. The primary aim is to reduce the time of inpatient hospitalisation while continuing to provide a high quality package of care for patients. The advantages of enhanced recovery include early mobilisation with an associated reduction in thromboembolic events, shorter hospital stay and, as a consequence, reduced risk of hospital acquired infections as well as reduced hospital costs.1
In 2007 Ranawat and Ranawat described a fast track pathway using regional analgesia, multimodal pain control with local periarticular injections and accelerated rehabilitation.2 This could lead to a painless recovery, a new concept for arthroplasty surgery. This type of enhanced recovery pathway is now the normal path of treatment for many patients undergoing primary arthroplasty in the UK. Individual centres follow locally agreed enhanced recovery pathways but all have the aim of optimising analgesia, allowing early mobilisation and timely discharge.
Enhanced recovery for patients undergoing primary lower limb arthroplasty has been in place in our hospital since 2010. The pathway aims for early mobilisation and discharge on day 3. In order to aid mobilisation on the day of surgery, regional nerve blocks are avoided, multimodal analgesia is used and tranexamic acid is given at induction to reduce blood loss.3–5 Drains are not used. Despite this, some patients are slow to mobilise and discharge is consequently delayed. The multidisciplinary team felt that pain was the biggest contributor to poor postoperative recovery and reluctance to mobilise. The primary aim of this study was to investigate the analgesia requirements and pain levels of primary arthroplasty patients on the enhanced recovery pathway. Secondary aims were to identify a group of patients in whom additional analgesia would be of benefit and to assess reasons for delayed discharge.
Methods
This was a prospective, multidisciplinary cohort study carried out from January to March 2012. The study was devised by the orthopaedic and anaesthetic teams in conjunction with all relevant allied health professionals. The study was discussed and registered with the local clinical effectiveness unit.
All patients undergoing primary total hip (THA) and total knee arthroplasty (TKA) in the lower limb arthroplasty unit at one hospital who were eligible for the enhanced recovery pathway were included in the study. Patients with an ASA (American Society of Anesthesiologists) grade of <3 and a body mass index of <40kg/m2 are automatically put in the enhanced recovery pathway. Patients attended for preoperative education, as is standard practice, in advance of their admission date. They were informed that following surgery they would experience some pain, advised about their postoperative rehabilitation and instructed on pain management in order to manage their expectations.
Operations were carried out by six lower limb arthroplasty consultants, two knee surgeons and one fellow. All patients were given central neuraxial blockade (spinal) or general anaesthesia. Epidural anaesthesia was avoided to allow early mobilisation. Spinal anaesthesia consisted of 0.5% bupivacaine, plain or heavy as per anaesthetist’s preference, to a total volume of 2.5–3ml including 300μg (0.3ml) diamorphine. Intravenous (IV) flucloxacillin 1g, gentamicin 80mg and tranexamic acid 1g were administered in the anaesthetic room prior to surgery commencing. Patients with a penicillin allergy received IV teicoplanin 400mg and tranexamic acid was withheld in patients with a contraindication.
Surgical technique was the default technique of the operating surgeon. Local infiltration of bupivacaine 0.125% (80ml) or 0.25% (40ml) was injected either into the capsule prior to cementing or into the fascia and subcutaneous tissue at the time of closure depending on surgeon preference. Drains were not used. Patients were prescribed regular paracetamol 1g four times a day in conjunction with a weak opiate four times a day (codeine 30mg or tramadol 100mg) and diclofenac 50mg three times a day as dictated by patient co-morbidity. Additionally, morphine (5–10mg subcutaneously or 10–20mg orally) and weak opiates (30mg) were available as required.
A proforma detailing patient demographics, anaesthetic technique, analgesia prescribed and use of perioperative local anaesthetic infiltration was completed by the anaesthetist in theatre at the time of surgery. The proforma was sent back to the ward with the patient and placed with the observation charts at the end of the patient’s bed. Patients were helped to mobilise postoperatively on the ward on the day of surgery and daily thereafter until completion of a structured physiotherapy programme. At the time of mobilisation with the physiotherapist, the patient was asked to rank his or her pain on a visual analogue scale of 0–10.6
At the point of discharge, the length of stay and any reason for delayed discharge were recorded. The drugs card was analysed. Regular analgesia administered and any additional analgesia requirements were recorded. All strong opiate requirements were converted to the IV equivalent for comparison purposes.
Data were collated using Excel® (Microsoft, Redmond, WA, US). Statistical analysis was carried out using the Social Science Statistics website (http://www.socscistatistics.com/). A Mann–Whitney U test was used to assess the difference in pain scores for those patients undergoing TKA and THA with and without the use of NSAIDs. The number of patients in each group needing to use strong opiate analgesia was analysed using a chi-squared test. The volume of strong opiate analgesia required for each patient was converted to an IV equivalent and the two groups were compared at day 0, day 1 and day 2 using a Mann–Whitney U test.
Results
One hundred and two patients had a proforma. Six of these were incomplete and were therefore excluded. Of the 96 patients included in the study, 34 underwent THA and 62 TKA. Patient demographics are shown in Table 1.
Table 1.
Patient demographics
| THA (n=34) | TKA (n=62) | |
|---|---|---|
| Mean age (range) | 72 yrs (53–89 yrs) | 71 yrs (44–89 yrs) |
| Male-to-female ratio | 16:18 | 20:42 |
| Median ASA grade (range) | 2 (1–3) | 2 (1–3) |
THA = total hip arthroplasty; TKA = total knee arthroplasty; ASA = American Society of Anesthesiologists
Sixty-five per cent of all arthroplasty patients were discharged on or before day 3. All patients received either general anaesthesia or central neuraxial blockade (spinal); no patients received both. Tranexamic acid was given to 90% of patients, 9% had a contraindication preventing the use of tranexamic acid and 82% received local infiltration into the joint perioperatively. Only 58% of all arthroplasty patients received non-steroidal anti-inflammatory drugs (NSAIDs) as part of their multimodal analgesia.
Of the THA patients, 12 (34%) were classified as delayed discharge (>3 days) cases. Pain was an issue for four (30%) of these patients and problems with homecare after discharge was also a significant contributor for four patients (30%). Other reasons for delay in discharge included leaking wound (n=1), low haemoglobin (n=1) and re-warfarinisation (n=1). Patients undergoing THA had median pain scores of 5 or less throughout the postoperative period. The median length of stay was 3 days. There was no correlation between the analgesia regime and pain scores for patients undergoing THA.
For the TKA patients, 23 (37%) had a delayed discharge. Pain was the most common reason for delayed discharge in 13 (57%) of these patients. Other reasons included homecare (n=4, 18%), leaking wound (n=3, 13%) and minor medical conditions (n=3, 13%). The median length of stay was 3 days. The median pain scores for patients undergoing TKA were all above 5.
Patients undergoing THA had lower pain scores than those undergoing TKA. Patients who had TKA and did not receive NSAIDs had the highest pain scores throughout. These were significantly higher (day 0, p<0.01; day 1, p<0.001; day 2, p<0.01) than for those TKA patients who did receive NSAIDs (Fig 1). There was no difference in pain scores between THA patients, with or without NSAID use.
Figure 1.
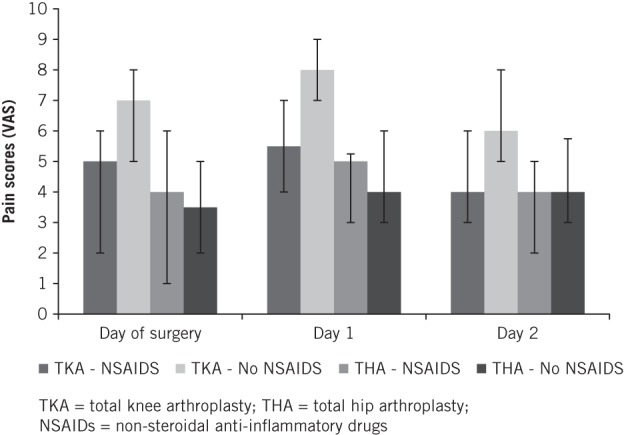
Median postoperative visual analogue scale pain scores for primary arthroplasty patients.
This study showed no correlation between local infiltration and pain scores for either THA or TKA groups. Infiltration technique was surgeon dependent and not a standardised procedure from which conclusions can be drawn in this study, and this therefore warrants further investigation.
There was no significant difference in the number of patients requiring opiates in each group (THA with NSAIDs vs THA without NSAIDs and TKA with NSAIDs vs TKA without NSAIDs). However, there was a significant difference in the volume of opiates required (Table 2).
Table 2.
Mean volume of strong opiate use (intravenous equivalent)
| Day 0 Mean (range) | Day 1 Mean (range) | Day 2 Mean (range) | Day 3 Mean (range) | |
|---|---|---|---|---|
| THA without NSAIDs | 1.87mg (0–12mg) | 2.43mg (0–23mg) | 0.55mg (0–10mg) | 0.07mg (0–3mg) |
| THA with NSAIDs | 2.62mg (0–20mg) | 1.86mg (0–13mg) | 0.15mg (0–1mg) | 0.15mg (0–1mg) |
| TKA without NSAIDs | 8.16mg (0–44mg) | 9.07mg (0–30mg) | 2.01mg (0–10mg) | 0.00mg (0–0mg) |
| TKA with NSAIDs | 4.63mg (0–25mg) | 4.72mg (0–15mg) | 0.12mg (0–3mg) | 0.00mg (0–0mg) |
THA = total hip arthroplasty; NSAIDs = non-steroidal anti-inflammatory drugs; TKA = total knee arthroplasty
TKA patients without NSAIDs had significantly higher strong opiate volume requirements on day 0 (p<0.05), day 1 (p<0.05) and day 2 (p<0.05) than those patients with NSAIDs (Fig 2). There was no difference in strong opiate requirements for THA patients with or without NSAIDs.
Figure 2.
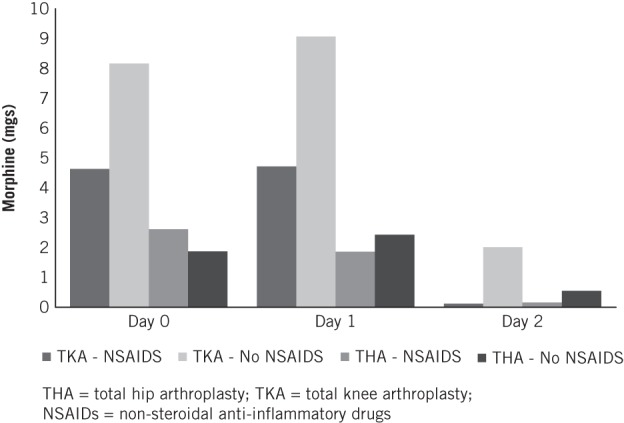
Mean volume of opiates used after primary arthroplasty surgery (intravenous equivalent)
Discussion
Pain scores for TKA were higher than those for THA. This is in keeping with our clinical experience. In our institution, pain scores greater than 5 are accepted as being severe. All the TKA patients had pain scores of 5 or more and those patients treated without the use of NSAIDs had significantly higher scores.
Lamplot et al7 reiterated the advantages of multimodal analgesia proposed by Ranawat and Ranawat.2 Their randomised controlled trial (RCT) of multimodal analgesia versus opiate patient controlled analgesia (PCA) concluded that multimodal pain management decreases narcotic usage, improves pain scores, increases satisfaction and enhances early recovery.7
Traditionally, in our unit, there has been an avoidance of the use of NSAIDs to reduce the risks of acute kidney injury. NSAIDs are effective analgesics but caution is given to use in vulnerable populations such as those at risk of renal injury (chronic kidney disease stage 3 and above), the elderly and those with concomitant nephrotoxic medication. This study shows that there is a significant benefit to using NSAIDs, namely reduced opiate requirements and lower pain scores; we suggest they should be considered and used with caution rather than excluded completely.
These results are in keeping with those of Buvanendran et al, who found that the administration of perioperative NSAIDs as part of a multimodal analgesia regime reduced pain scores, opiate requirements and vomiting levels, and led subsequently to improved range of motion after TKA.8 There are no reports in the literature of systemic effects of NSAIDs when administered into the joint and it is therefore worth considering whether local NSAIDs should be used instead.
Ketorolac is a locally acting NSAID. It is thought to reduce the local inflammatory response and work as an adjunct to local anaesthesia. Andersen et al reported a significant reduction in narcotic use and length of stay for patients following THA who were treated with local infiltration and a bolus injection of ropivacaine, ketorolac and epinephrine compared with those with continuous epidural infusion.9 More recently, they have studied the effects of local anaesthetic infiltration with and without ketorolac.10 They concluded that the use of ketorolac in the local infiltration solution resulted in a significant reduction in IV morphine PCA consumption but there was no difference in length of stay.
The use of intra-articular local anaesthetic infiltration is becoming increasingly popular. In 2008 Kerr and Kohan11 reported a regime of local infiltration analgesia of bupivacaine, adrenaline and ketorolac. They studied 325 patients who had a bolus injection at the time of surgery and reinfusion of the mixture into the joint 15–20 hours postoperatively. Two-thirds of the patients had no opiate requirements; pain scores ranged from 0 to 3 and 71% of patients were discharged after one day. This proved to be a simple and highly effective treatment pathway.
At present, there are no RCTs comparing bolus intra-articular injection at time of surgery with a continuous infusion. Starks et al have reviewed the RCTs related to local infiltration in joint replacement and enhanced recovery.12 Of the 15 TKA studies, 11 used local infiltration in conjunction with PCA; all of the studies used either a continuous infusion of local anaesthetic or intra-articular reinjection within the first 24 hours. They all claim effective pain management but no two studies have comparable regimes.
In a double blind RCT of local anaesthetic infiltration (morphine, bupivacaine, ketorolac and adrenaline) into one knee during bilateral TKA, Fajardo et al showed patients have significantly reduced pain and mobilise earlier following periarticular infiltration.13 Unlike other studies, this study eliminates the subjective element of pain as patients acted as their own controls. The effect of local anaesthetic infiltration on pain scores was inconclusive in our study. Aiming to reduce patient pain without systemic effects is clearly an area that is a developing interest. However, further work is needed to quantify the optimum composition, volume and position of intra-articular injection.
Improving patient experience by dealing effectively with their pain aids recovery and early discharge. This study assesses postoperative pain on the enhanced recovery pathway but the concept of pain catastrophising suggests that in addition to analgesia, understanding a patient’s individual response to pain can help reduce pain levels. Pain catastrophising is conceptualised as a negative cognitive–affective response to anticipated or actual pain. In a review of papers reporting the psychological factors affecting outcomes in arthroplasty patients, Vissers et al found that for knee patients only, strong evidence was found that patients with pain catastrophising reported more pain postoperatively.14 This is not the case for THA.
Preoperative identification of patients with a higher pain response would allow us to act pre-emptively by trying to manage the patient’s pain and anxieties from the start of management, thereby improving patient satisfaction postoperatively. Granot et al noted that patients undergoing elective Caesarean section who had high preoperative pain scores with a heat stimulus also had higher postoperative pain scores.15 On the contrary, however, Lunn et al found no correlation between preoperative pain scores after heat stimulus and postoperative pain scores following TKA, and would not recommend clinical use.16 The overall prediction rate of postoperative pain using preoperative testing is 4–54% depending on the tests used.17 This large variation in results gives us no clear evidence on which to base our practice but this area of individual response to pain warrants further investigation.
Unfortunately, in this study, the data for day 3 are too small to draw valid conclusions. A proportion of patients had been discharged home and achieved enhanced recovery. Patients were discharged from physiotherapy once they had achieved their targets. One limitation of the visual analogue scale is the inability of some elderly or cognitively impaired patients to complete the score unaided. For simplicity and reproducibility, one operator was therefore chosen to help the patients complete the pain scores. As per usual practice in our trust, this was carried out by the physiotherapist. Once physiotherapy was complete (which is independent of pain scores), pain scores were no longer recorded and this is a limitation of the study.
Conclusions
Pain control and early discharge in patients after THA is acceptable. Unacceptably high pain scores in patients undergoing TKA without the use of NSAIDs have been demonstrated. The use of NSAIDs reduces pain scores and opiate requirements. NSAIDs are, however, not suitable for all patients. There should be focused intervention with this group of patients to improve postoperative pain scores and reduce length of stay.
Acknowledgements
We would like to thank Stockley, Hamer, Buckley, Wilkinson, Brown and Sutton for allowing the inclusion of their patients in this study. We are also grateful to H Rehmani, Foundation Trainee, who assisted in data collection.
References
- 1.Andersen SH, Husted H, Kehlet H. Economic consequences of accelerated care pathways in total knee-arthroplasty. Ugeskr Laeger 2009; 171: 3,276–3,280. [PubMed] [Google Scholar]
- 2.Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty 2007; 22(7 Suppl 3): 12–15. [DOI] [PubMed] [Google Scholar]
- 3.Wind TC, Barfield WR, Moskal JT. The effect of tranexamic acid on blood loss and transfusion rate in primary total knee arthroplasty. J Arthroplasty 2013; 28: 1,080–1,083. [DOI] [PubMed] [Google Scholar]
- 4.Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br 2011; 93: 39–46. [DOI] [PubMed] [Google Scholar]
- 5.Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee 2006; 13: 106–110. [DOI] [PubMed] [Google Scholar]
- 6.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res 2011; 63: S240–S252. [DOI] [PubMed] [Google Scholar]
- 7.Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014; 29: 329–334. [DOI] [PubMed] [Google Scholar]
- 8.Buvanendran A, Kroin JS, Tuman KJ et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA 2003; 290: 2,411–2,418. [DOI] [PubMed] [Google Scholar]
- 9.Andersen KV, Pfeiffer-Jensen M, Haraldsted V, Søballe K. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty. Acta Orthop 2007; 78: 180–186. [DOI] [PubMed] [Google Scholar]
- 10.Andersen KV, Nikolajsen L, Haraldsted V et al. Local infiltration analgesia for total knee arthroplasty: should ketorolac be added? Br J Anaesth 2013; 111: 242–248. [DOI] [PubMed] [Google Scholar]
- 11.Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery. Acta Orthop 2008; 79: 174–183. [DOI] [PubMed] [Google Scholar]
- 12.Starks I, Wainwright T, Middleton R. Local anaesthetic infiltration in joint replacement surgery: what is its role in enhanced recovery? ISRN Anaesthesiol 2011; 742927. [Google Scholar]
- 13.Fajardo M, Collins J, Landa J et al. Effect of a perioperative intra-articular injection on pain control and early range of motion following bilateral TKA. Orthopaedics 2011; 34: 354. [DOI] [PubMed] [Google Scholar]
- 14.Vissers MM, Bussmann JB, Verhaar JA et al. Psychological factors affecting the outcome of total hip and knee arthroplasty: a systematic review. Semin Arthritis Rheum 2012; 41: 576–588. [DOI] [PubMed] [Google Scholar]
- 15.Granot M, Lowenstein L, Yarnitsky D et al. Postcesarean section pain prediction by preoperative experimental pain assessment. Anaesthesiology 2003; 98: 1,422–1,426. [DOI] [PubMed] [Google Scholar]
- 16.Lunn TH, Gaarn-Larsen L, Kehlet H. Prediction of postoperative pain by preoperative pain response to heat stimulation in total knee arthroplasty. Pain 2013; 154: 1,878–1,885. [DOI] [PubMed] [Google Scholar]
- 17.Werner MU, Mjöbo HN, Nielsen PR, Rudin A. Prediction of postoperative pain: a systematic review of predictive experimental pain studies. Anaesthesiology 2010; 112: 1,494–1,502. [DOI] [PubMed] [Google Scholar]


