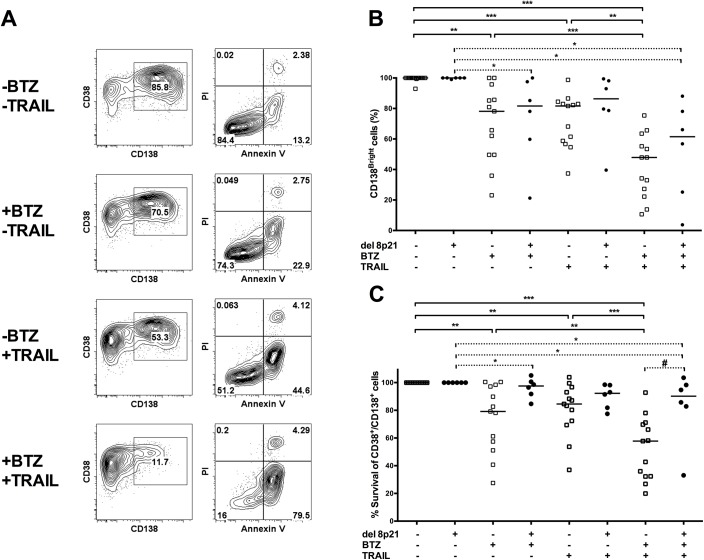
Fig 4. Patients with del(8)(p21) are less sensitive to bortezomib and TRAIL mediated apoptosis.
CD138 expression levels and Annexin V/PI staining was performed to assess viable, apoptotic and dead cells. Mononuclear cells were separated by Lymphoprep from fresh bone marrow aspirates of MM patients followed by CD138 positive magnetic selection. Cells were incubated overnight with or without 10 nM bortezomib followed by incubation with or without 250 ng/ml soluble TRAIL/APO2L for 6 hours. Thereafter, cells were stained with anti-CD38, anti-CD138, anti-Annexin V antibodies and PI. (A) Representative patient data displaying relative changes in CD38+/CD138Bright cell population (left panel) and Annexin V/PI (right panel) upon bortezomib and/or soluble TRAIL/APO2L treatment. (B) Relative changes in the CD38+/CD138Bright cell population of 13 MM patients without del(8)(p21) and 6 MM patients with del(8)(p21) upon bortezomib and/or soluble TRAIL/APO2L treatment is displayed. Amount of CD38+/CD138Bright cells of each untreated patient MM cells is considered as 100%. (C) Relative percentage of live MM cells (Annexin V- and PI- cells) of 13 MM patients without del(8)(p21) and 6 MM patients with del(8)(p21) is displayed. Percentage of untreated live MM cells is considered as 100%. Statistical analysis of bortezomib and/or TRAIL treatment is assessed with Wilcoxon rank test in patients with or without del(8)(p21). * P<0.05, ** P<0.01, *** P<0.001. Mann-Whitney test was performed to compare patients groups with and without del(8)(p21). # P<0.05. Median values for each group are displayed in B and C.