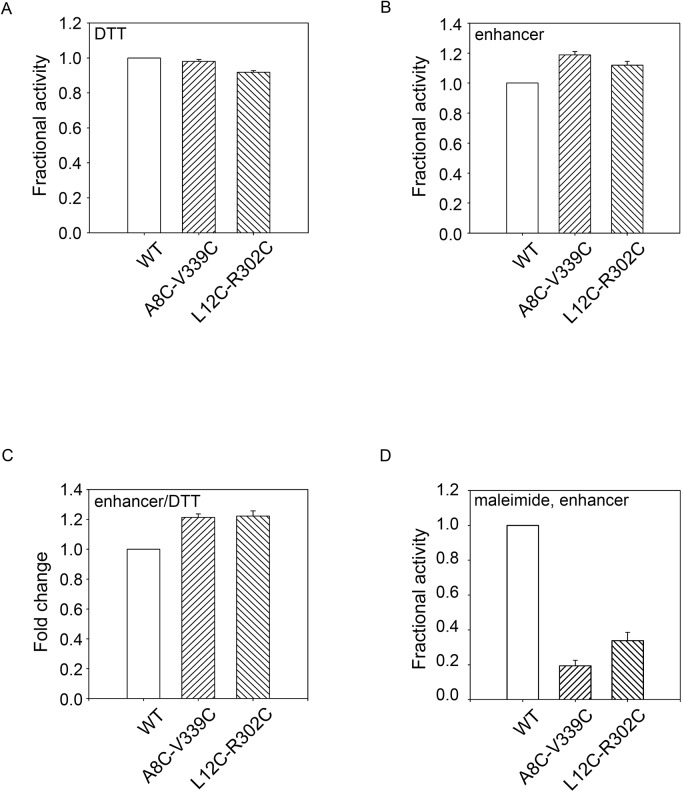
Fig 5. Disulfide stabilization under relaxed biochemical conditions.
The PrtS cysteine mutants were refolded and assessed functionally in reducing and oxidizing conditions and activity was assessed at pH 7.5 at 55°C. A, activity assays of the PrtS proteins refolded in the presence of DTT are shown and normalized to the activity observed with the wildtype protein. B, activity assays of the PrtS proteins refolded in the presence of a disulfide-enhancing additive are shown and normalized to the activity observed with the wildtype protein. C, the fold change between reducing and oxidizing conditions, A and B, is shown for the wildtype and mutant proteins. D, the PrtS protein were pre-incubated with maleimide and refolded as in C. Activity is shown normalized to the wildtype. Data shown are mean ± standard deviation from at least three independent experiments. Relative activity values were derived from the mean reaction velocities under steady state conditions with wildtype PrtS activity normalized to 1.0.