Abstract
Purpose
Pseudomonas aeruginosa keratitis is a sight-threatening complication of contact lens wear, yet mechanisms by which lenses predispose to infection remain unclear. Here, we tested the hypothesis that tear fluid at the posterior contact lens surface can lose antimicrobial activity over time during lens wear.
Methods
Daily disposable lenses were worn for 1, 2, 4, 6 or 8 h immediately after removal from their packaging, or after presoaking in sterile saline for 2 days to remove packaging solution. Unworn lenses were also tested, some coated in tears “aged” in vitro for 1 or 8 h. Lenses were placed anterior surface down into tryptic soy agar cradles containing gentamicin (100µg/ml) to kill bacteria already on the lens, and posterior surfaces inoculated with gentamicin-resistant P. aeruginosa for 3 h. Surviving bacteria were enumerated by viable counts of lens homogenates.
Results
Posterior surfaces of lenses worn by patients for 8 h supported more P. aeruginosa growth than lenses worn for only 1 h, if lenses were presoaked prior to wear (~ 2.4-fold, p = 0.01). This increase was offset if lenses were not presoaked to remove packaging solution (p = 0.04 at 2 h and 4 h). Irrespective of presoaking, lenses worn for 8 h showed more growth on their posterior surface than unworn lenses coated with tear fluid that was “aged” for 8 h vitro (~8.6-fold, presoaked, p = 0.003: ~ 5.4-fold from packaging solution, p = 0.004). Indeed, in vitro incubation did not impact tear antimicrobial activity.
Conclusions
This study shows that post lens tear fluid can lose antimicrobial activity over time during contact lens wear, supporting the idea that efficient tear exchange under a lens is critical for homeostasis. Additional studies are needed to determine applicability to other lens types, wearing modalities, and relevance to contact lens-related infections.
Keywords: contact lenses, Pseudomonas aeruginosa survival, tear fluid, posterior lens surface, lens packaging solution, daily wear
Pseudomonas aeruginosa can cause sight-threatening microbial keratitis (MK), which remains the most severe complication of contact lens wear.1 All contact lens wearing modalities, from daily disposable to extended wear, carry a risk.2–5 Because improvements in oxygen permeability of contact lens materials have not reduced disease incidence,4–7 suggesting mechanisms beyond hypoxia are involved.8, 9 An untested hypothesis in the field is that a lack of tear exchange is responsible. This idea is based on the assumption that the ocular surface needs adequate tear exchange to avoid infection, and could explain why soft contact lenses, which are known to provide limited tear exchange,10, 11 are associated with a greater risk of infection than rigid gas permeable (RGP) lenses.2, 3 Indeed, inadequate tear exchange is likely to trap debris and metabolic by-products, in addition to microbes and their toxins. Since tear fluid components come from multiple glands and cell types around the ocular surface, inadequate tear exchange/tear mixing under a lens could alter tear composition at the corneal surface. That could in turn compromise its various homeostatic functions, which include direct antimicrobial activity,12, 13,14 and its recently demonstrated capacity to protect the corneal epithelium against bacterial virulence mechanisms independently of its antimicrobial activity.12, 15–18
While the impact of contact lens wear on the composition of tear fluid collected from the conjunctival sac has been studied,19, 20 (Review21) how tear fluid between the lens and cornea (post-lens tears) is affected in composition or capacity to protect the cornea against bacteria is not known. Underlying this gap in knowledge central to our understanding of why contact lens wearers are predisposed to infection, is the technical difficulty of collecting and/or analyzing the extremely small volume of fluid that sits between a soft lens and the cornea.
Here, we used worn contact lenses as a method for sampling the tear fluid trapped between the lens and the corneal surface. While still on the back surface of the lens, we tested the fluid for its antimicrobial activity against P. aeruginosa in vitro. The results showed that antimicrobial activity was lower when lenses were worn for a period of 8 h than if the lens was worn for only 1 h, but that this was only significant if the lens was presoaked to remove the packaging solution before the wearing period. Antimicrobial activity was retained if tear fluid collected from the conjunctival sac was incubated for 8 h in vitro, suggesting mechanisms involved in loss of activity under a lens may be complex.
METHODS
Human Subjects
Ten healthy participants were recruited. Four female subjects participated in lens wearing experiments, and six others (4 female and 2 male) participated in tear fluid collection. Participant ages ranged from 20 to 42 years. Informed consent was obtained from all participants, and all procedures were approved by the Committee for the Protection of Human Subjects, University of California, Berkeley. This research adheres to the tenets of the Declaration of Helsinki.
Lens wearing experiments involved experienced contact lens wearers with 5 to 10 years of lens wear. Prior to beginning the study, each participant was examined by an Optometrist to confirm the absence of anterior surface abnormalities or disease. Commercially available, daily disposable, hydrogel contact lenses (Omafilcon A, H2O 60%) were purchased for use in this study, and lens fit was optimized for each patient. Lenses were worn by participants for 1, 2, 4, 6 or 8h either immediately after removal from their blister packaging solution, or after pre-soaking in 50ml of sterile saline (sodium chloride 0.9% w/v; Winchester Laboratories Germany) for 2 days to remove packaging solution. Contact lenses were fitted in the morning between 8 – 10 am to reflect normal wearing schedules. Prior to lens insertion into the eyes, lenses were shaken gently to remove any excessive fluid either from the blister packaging solution or from the sterile saline. After wearing lenses for the specified duration, participants removed the lenses from their eyes while wearing powder-free sterile gloves. Investigators collected lenses immediately after removal from the eyes. Each participant returned to the laboratory for lens removal on the same day after the required wearing time resulting in two visits per day. Participant visits were not scheduled on consecutive days, and lens-wearing times for participant visits were randomized.
To test effects of lens curvature, a flat (base curve 8.5) and a steep (base curve 9.0) hydrogel lens pair (Etafilcon A, H2O 58%, same manufacturer) were worn for 8 h by a participant. After 8 h of wear, lenses were removed as described previously. Two participants were involved, and each repeated the experiment after 5 days using new lenses and alternating base curve assignment.
While tear collection participants included lens wearers and non-wearers, the lens wearers were asked to discontinue lens wear for at least one day prior to tear collection. Tear fluid was collected by placing a micro-capillary tube at the lower conjunctival sac.22 Approximately one hundred microliters of tear fluid was collected over approximately 30 minutes on each occasion and the collected tears were stored at −80°C prior to use in experiments. Tears were pooled prior to use in experiments.
Bacteria
Pseudomonas aeruginosa strain PAO1 complemented with plasmid pJNEO5 was used. Plasmid pJNEO5 encodes gentamicin resistance to allow bacterial selection after inoculation of previously worn or unworn lenses. Bacterial inocula were prepared by first growing bacteria on trypticase soy agar (TSA) plates supplemented with gentamicin (100µg/mL) for approximately 16 h at 37°C. Isolated colonies were then suspended in PBS to an absorbance of 0.1 at 650nm (~1 × 108 colony forming units [CFU]/mL), and then diluted 100-fold to a concentration of ~1 × 106 CFU/ml (~1000 CFU/µl) for use in experiments.
Determination of Post-lens Antimicrobial Activity
After removal from the eye, lenses were placed with their anterior surface facing down into TSA cradles supplemented with gentamicin (100µg/ml) to suppress the growth of any bacteria transferred to the lens during wear or handling (Fig. 1A). Prior to lens insertion, the cradle well was filled with PBS (~200µl) to minimize drying of the anterior lens surface which was placed face down in the cradle. The center of the posterior lens surface, previously in close association with the cornea and facing upwards in the cradle, was carefully inoculated with 1µl of bacterial suspension (~1000 CFU). This very small volume of inoculum was used to minimize any "wash-out" effect of the tear fluid at the posterior surface. The cradle Petri dish was sealed with Parafilm and placed in a larger Petri dish filled with PBS (2mL) (Fig. 1B) to further minimize dehydration, and was incubated for 3 h at 35°C. Surviving bacteria were then enumerated by viable count of the lens homogenate. Briefly, each lens was transferred into a glass homogenizer using sterile tweezers and homogenized in PBS (1mL) containing Triton X-100 (0.25% vol./vol.) which prevents bacterial clumping without affecting bacterial viability.12 The homogenate was plated on TSA supplemented with gentamicin 100µg/mL to select only inoculated bacteria (P. aeruginosa PAO1-pJNEO5). Unworn lenses taken directly from the original packaging, or pre-soaked in saline for 2 days, were used as controls.
Figure 1.
Experimental model. (A) Modified TSA plate holding worn contact lenses with the posterior surface facing upwards. TSA was supplemented with gentamicin (100µg/mL) to suppress bacteria associated with lens wear. (B) Modified TSA plate was wrapped in Parafilm and placed in a larger dish with PBS to minimize dehydration during incubation (3h).
In other experiments, tear fluid was collected from the conjunctival sac of non-lens wearing eyes, and then incubated in vitro at 35°C for either 1 h or 8 h to “age” it before adding it to unworn contact lenses. Some lenses were treated immediately after removal from the packaging solution, while others were first pre-soaked in saline for 2 days. Aged tear fluid was added by placing the contact lens and tear fluid in a 250µL Eppendorf tube to minimize the amount of tear fluid required. The anterior surface of the lens conformed to the cylindrical shape of the tube. Tear fluid (150µL) was added to the posterior surface of the lens, and the Eppendorf tube recapped and positioned horizontally so that the tear fluid covered the center zone of the posterior lens. After soaking the lenses in 1 h or 8 h aged tears for ~ 5 – 10 minutes, the lenses were removed from the pool of tears, and shaken gently to remove any excessive tear fluid before being placed into TSA cradles and the posterior surface (only) challenged with P. aeruginosa.
Statistical Analysis
Bacterial viability was expressed as CFU/lens and the data expressed as the mean (+/−SEM). Based on a pilot study, a sample size of four was required for each group to detect 2-fold differences in bacterial recovery between each condition (power of 95%). The statistical significance of differences between groups was determined using the Mann–Whitney U Test since the data were non-parametric. P values of <0.05 were considered significant.
RESULTS
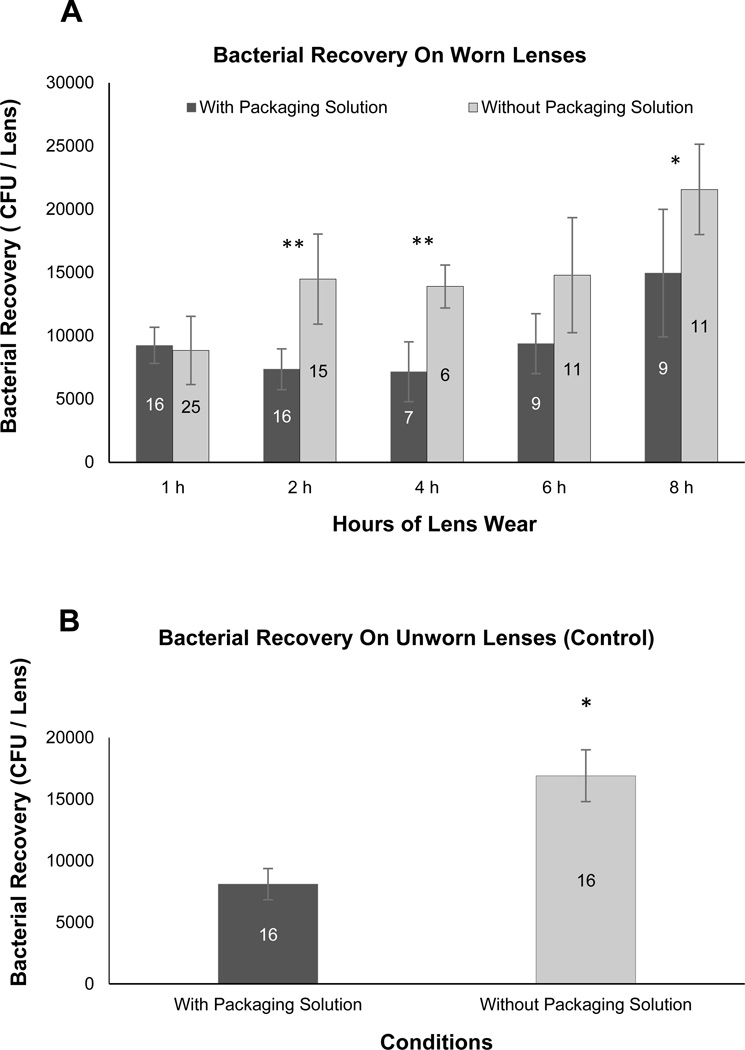
Contact lenses (Omafilcon A) were worn by healthy volunteers for times varying from 1h to 8h, and the antimicrobial activity of the posterior lens surface (containing post-lens tear fluid) against P. aeruginosa was determined using the 3h TSA cradle assay (see Methods). The results showed a reduction in the antimicrobial activity of the fluid at the posterior lens surface with time. This was statistically significant, as shown by comparison of 1h and 8h of wear for lenses presoaked to remove packaging solution prior to wear (Fig. 2A). While lenses worn directly after removal from their original packaging solution also showed a reduction in posterior lens surface antimicrobial activity over 8h, this was not statistically significant. Importantly, at both 2h and 4h of wear, the posterior surface of lenses worn directly after removal from their packaging solution was significantly more antimicrobial than presoaked lenses (Fig. 2A). This correlated with differences between them in their antimicrobial activity when they were not worn (Fig. 2B). Surprisingly, at the earlier (1h wear) time point they were similarly antimicrobial. Another surprising finding was that lenses of different curvature (Etafilcon A; worn for 8h directly from the packaging solution), had similar post-lens antibacterial activity (data not shown).
Figure 2.
(A) Antimicrobial effect of posterior lens surfaces after different times of lens wear. Daily disposable contact lenses (Omafilcon A) were worn for various times up to 8h either directly after removal from packaging solution (black bars), or after presoaking in sterile saline for 2 days (gray bars). Data show the mean (+/−SEM) number of P. aeruginosa recovered per lens after 3 h exposure to these worn lenses in vitro after inoculation with ~103 cfu P. aeruginosa PAO1 + pJNEO5 (gentamicin resistant, see Figure 1 and Materials and Methods). *p = 0.01, 1 h versus 8h lens wear (presoaked, no packaging solution, gray bars). **p = 0.04 (2h) and p = 0.04 (4h) lens wear comparing with and without packaging solution. (B) Antimicrobial activity of posterior surfaces of unworn contact lenses. Daily disposable contact lenses were inoculated with ~103 cfu of P. aeruginosa PAO1 + pJNEO5 either directly after removal from the original packaging solution (black bars), or after presoaking in sterile saline for 2 days (gray bars). Data show the mean (+/− SEM) number of P. aeruginosa recovered per lens after 3h exposure in vitro. *p = 0.002. Numbers on the bars indicate the number of lenses used for each data point.
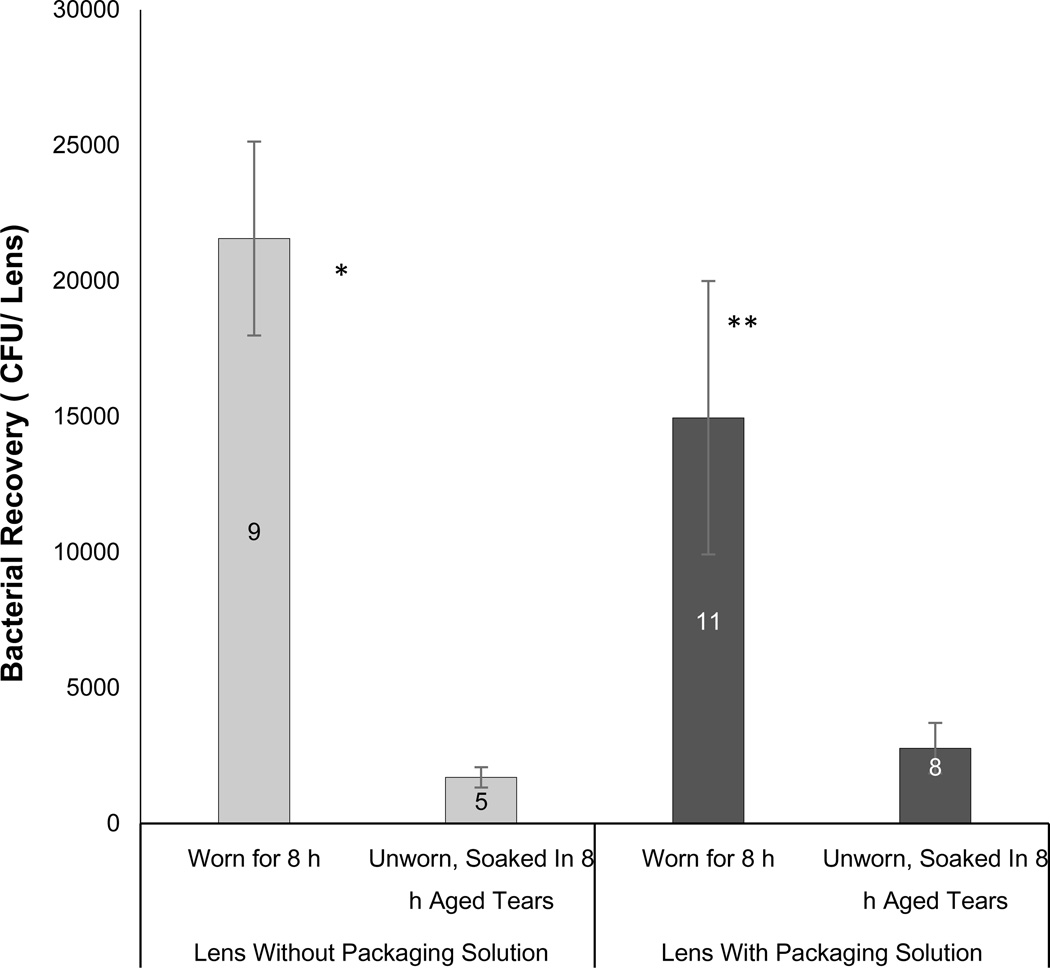
Potential mechanisms for reduced antibacterial activity of the fluid at the posterior lens surfaces with longer wear include simple degradation of active tear components over time, enabled by stagnation. To explore that possibility, antibacterial activity of fluid on the posterior surface of 8h worn lenses (Omafilcon A) was compared to unworn lenses soaked in tear fluid aged in vitro for 8h. The latter was done by placing tear fluid collected using a microcapillary tube in an incubator at 37°C for 8 h (versus 1 h) before soaking lenses in it. For lenses presoaked to remove packaging solution, the 8h worn lenses were 8.6-fold less antimicrobial against P. aeruginosa than unworn lenses of the same type soaked tear fluid that had been aged in vitro for 8h (Fig. 3, p = 0.003). Similarly, if the same lens type was tested directly after removal from the packaging solution, the 8h worn lenses were 5.4-fold less antimicrobial against P. aeruginosa than unworn lenses soaked tear fluid that had been aged in vitro for 8h (Fig. 3, p = 0.004). In control experiments (not shown), 1h or 8h in vitro aged tear fluid was compared on unworn lenses with and without packaging solution. Results verified that the antimicrobial activity of 1h aged tears on posterior lens surfaces was not significantly different from that of 8h aged tears.
Figure 3.
Comparison of post-lens antimicrobial activity between Omafilcon A lenses worn for 8h versus the same lens type unworn and soaked in human tear fluid that had been previously aged for 8h in vitro. Lenses were inoculated with ~103cfu of P. aeruginosa PAO1 + pJNEO5 and incubated for 3 h before recovery of viable bacteria from lens homogenates. Prior to bacterial exposure, lenses were either worn for 8h, or were unworn and soaked in human tear fluid previously aged for 8h in vitro. Lenses were used directly after removal from original packaging solution (black bars), or after presoaking in sterile saline for 2 days (gray bars). Data show mean (+/− SEM) number of P. aeruginosa recovered per lens after 3h in vitro. Worn lenses were significantly less antimicrobial than unworn lenses soaked in tear fluid for both presoaked lenses (*p = 0.003) and lenses used directly from their packaging solution (**p = 0.004). Numbers on the bars indicate the number of lenses used for each data point.
DISCUSSION
The data presented in this study showed that the antimicrobial activity of tear fluid against the posterior (back) surface of a worn contact lens could decline during 8h of daily wear, an effect partially reduced by the lens packaging solutions at specific time points. In contrast, tear fluid collected from the conjunctival sac retained its antimicrobial activity when incubated in vitro for the same period of time.
Various potential mechanisms could explain why antimicrobial activity of tear fluid under a lens was reduced with increasing wear time but tear fluid incubated in vitro retained its antimicrobial activity. One is that loss of antimicrobial activity of tear fluid requires factors present in vivo, e.g. proteases made by the cornea, to degrade the active ingredients. An alternative would be that active ingredients are adsorbed onto the lens and become inactivated as a result. Indeed, reduced tear exchange or stagnation in vivo could allow increased time for binding, exclusion, and/or neutralization of tear antimicrobials via lens material(s),23 and/or via compartmentalization of tears causing concentration of factors that bind or degrade tear antimicrobials, e.g. exfoliated cells and debris, albumin, and proteases.24 Under those circumstances, lack of replenishment of tear antimicrobials from poor tear exchange could contribute to the reduced antimicrobial activity observed. Yet another possibility is that tear fluid collected from the conjunctival sac using a microcapillary tube, as done for the tear “aging” experiments, differs from tear fluid collected from the corneal surface using a lens, and that its antimicrobial ingredients are more stable. Indeed, the conjunctival sac collection method would collect mostly reflex tears, while fluid collected using a lens might be more similar to basal, or possibly even closed eye type, tears. Basal, reflex and closed eye tear fluid can differ in their composition.25, 26 Further, the pooling of tears and storage at −80°C needed to perform the in vitro tear “aging” experiments, might have altered the properties of tears compared to in situ. Whatever the case, the finding that tear fluid resulting from the two different methods both had antimicrobial activity at the outset, but only one had reduced activity after 8 hours, is an informative finding that provides us with directions for further mechanistic studies aimed at determining how and why tear fluid behind a lens loses antimicrobial activity.
Since dampening of posterior lens surface anti-Pseudomonal activity occurred within 8h of wear, changes favoring P. aeruginosa virulence could occur within a daily wear modality, and could be further enhanced by overnight or extended wear which show an increased risk of infection.3, 4, 27 That could allow for the greater persistence of P. aeruginosa, allowing more time for bacterial adaptation to the post-lens environment to favor phenotypes more capable of causing infection.28, 29 Prolonged exposure to P. aeruginosa, or its antigens, in the posterior lens environment could also influence corneal epithelial susceptibility to bacterial toxins or virulence mechanisms via expression of inflammatory mediators, infiltration of phagocytes, and other innate defense responses,8, 27, 30 and thereby contribute to the pathogenesis of P. aeruginosa keratitis and other contact lens-related complications involving P. aeruginosa or its antigens, e.g. CLARE (Contact Lens Acute Red Eye) and IK (Infiltrative Keratitis).27, 31
Not pre-soaking the lens partially offset the loss of antimicrobial activity at the posterior lens surface for the first few hours of wear. This result most likely reflects antimicrobial activity of residual packaging solution introduced with the lens. Indeed, our data confirmed that the packaging solution did have antimicrobial activity in vitro against the P. aeruginosa strain used. These findings show that packaging solutions containing antimicrobials to suppress the growth of microbes within the packaging container, might also be beneficial for controlling microbial viability at lens surfaces after the lens is placed on the eye.
In summary, the data presented in this report showed that post-lens tear fluid antimicrobial activity could decay over time during contact lens wear. Since conjunctival sac collected tear fluid retained its antimicrobial activity over the same time period when incubated in vitro, it is likely that additional in vivo factors are involved in the mechanism for loss of activity under a lens. This phenomenon was less significant for lenses not soaked prior to placing them in the eye, supporting the possibility that packaging solutions could be used to reduce microbial viability under a lens in vivo.
Contact lens-related infection is a vision-threatening disease impacting otherwise healthy patients. Its incidence has not changed since the introduction of soft contact lenses more than four decades ago, despite a plethora of products developed to address it. Contributing to this problem has been a lack of basic research aimed at understanding pathogenesis. The results presented in this report supporting the idea that there are changes to the post lens tear film favoring microbial survival, and suggest strategies for rectifying the problem. Since this was a small study with limited sample size, only two lens types, and one strain of a single pathogen, a larger clinical study is warranted to determine if these findings are applicable to other lenses (and lens packaging solutions), wearing modalities, other microbes, and a larger population.
ACKNOWLEDGMENTS
Supported by the National Institutes of Health EY011221 (SMJF), the American Optometric Foundation Vistakon Research Grant (YW/SMJF), and an Australian National Health and Medical Research Council C. J. Martin Fellowship (YW). Dr. Fleiszig is a paid consultant for Allergan Inc., Irvine CA. That work is unrelated to the content of this manuscript.
REFERENCES
- 1.Edwards K, Keay L, Naduvilath T, Snibson G, Taylor H, Stapleton F. Characteristics of and risk factors for contact lens-related microbial keratitis in a tertiary referral hospital. Eye (Lond) 2009;23:153–160. doi: 10.1038/sj.eye.6702953. [DOI] [PubMed] [Google Scholar]
- 2.Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG, Geerards AJ, Kijlstra A. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–185. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Keay L, Edwards K, Naduvilath T, Dart JK, Brian G, Holden BA. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Stapleton F, Keay L, Edwards K, Holden B. The epidemiology of microbial keratitis with silicone hydrogel contact lenses. Eye Contact Lens. 2013;39:79–85. doi: 10.1097/ICL.0b013e3182713919. [DOI] [PubMed] [Google Scholar]
- 5.Dart JK, Radford CF, Minassian D, Verma S, Stapleton F. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115:1647–1654. doi: 10.1016/j.ophtha.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Stapleton F, Edwards K, Keay L, Naduvilath T, Dart JK, Brian G, Holden B. Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology. 2012;119:1516–1521. doi: 10.1016/j.ophtha.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton F, Edwards K, Keay L, Naduvilath T, Dart JK, Brian G, Kaldor J, Holden B. Risk factors for severe microbial keratitis in daily wear contact lens users. Invest Ophthalmol Vis Sci. 2008;49 doi: 10.1016/j.ophtha.2012.01.052. E-abstract 4853. [DOI] [PubMed] [Google Scholar]
- 8.Evans DJ, Fleiszig SM. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am J Ophthalmol. 2013;155:961–970. doi: 10.1016/j.ajo.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DJ, Fleiszig SM. Microbial keratitis: could contact lens material affect disease pathogenesis? Eye Contact Lens. 2013;39:73–78. doi: 10.1097/ICL.0b013e318275b473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamara NA, Polse KA, Brand RJ, Graham AD, Chan JS, McKenney CD. Tear mixing under a soft contact lens: effects of lens diameter. Am J Ophthalmol. 1999;127:659–665. doi: 10.1016/s0002-9394(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 11.Miller KL, Polse KA, Radke CJ. Fenestrations enhance tear mixing under silicone-hydrogel contact lenses. Invest Ophthalmol Vis Sci. 2003;44:60–67. doi: 10.1167/iovs.02-0348. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003;71:3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. doi: 10.1016/j.biochi.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Beuerman RW, Huang L, Barathi A, Foo YH, Li SF, Chew FT, Tan D. Proteomic analysis of rabbit tear fluid: Defensin levels after an experimental corneal wound are correlated to wound closure. Proteomics. 2007;7:3194–3206. doi: 10.1002/pmic.200700137. [DOI] [PubMed] [Google Scholar]
- 15.Ni M, Evans DJ, Hawgood S, Anders EM, Sack RA, Fleiszig SM. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005;73:2147–2156. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNamara NA, Andika R, Kwong M, Sack RA, Fleiszig SM. Interaction of Pseudomonas aeruginosa with human tear fluid components. Current Eye Research. 2005;30:517–525. doi: 10.1080/02713680590969456. [DOI] [PubMed] [Google Scholar]
- 17.Mun JJ, Tam C, Evans DJ, Fleiszig SM. Modulation of epithelial immunity by mucosal fluid. Sci Rep. 2011;1:8. doi: 10.1038/srep00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mun J, Tam C, Chan G, Kim JH, Evans D, Fleiszig S. MicroRNA-762 is upregulated in human corneal epithelial cells in response to tear fluid and Pseudomonas aeruginosa antigens and negatively regulates the expression of host defense genes encoding RNase7 and ST2. PLoS One. 2013;8:e57850. doi: 10.1371/journal.pone.0057850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kijlstra A, Polak BC, Luyendijk L. Transient decrease of secretory IgA in tears during rigid gas permeable contact lens wear. Curr Eye Res. 1992;11:123–126. doi: 10.3109/02713689209000062. [DOI] [PubMed] [Google Scholar]
- 20.Farris RL. Tear analysis in contact lens wearers. CLAO J. 1986;12:106–111. doi: 10.1097/00140068-198604000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Mann A, Tighe B. Contact lens interactions with the tear film. Exp Eye Res. 2013;117:88–98. doi: 10.1016/j.exer.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Nagyova B, Tiffany JM. Components responsible for the surface tension of human tears. Curr Eye Res. 1999;19:4–11. doi: 10.1076/ceyr.19.1.4.5341. [DOI] [PubMed] [Google Scholar]
- 23.Carney FP, Morris CA, Milthorpe B, Flanagan JL, Willcox MD. In vitro adsorption of tear proteins to hydroxyethyl methacrylate-based contact lens materials. Eye Contact Lens. 2009;35:320–328. doi: 10.1097/ICL.0b013e3181becd3c. [DOI] [PubMed] [Google Scholar]
- 24.Sack RA, Sathe S, Beaton A, Nunes I. Tear proteases as a function of eye closure. Invest Ophthalmol Vis Sci. 1995;36(suppl.):995. [Google Scholar]
- 25.Markoulli M, Papas E, Petznick A, Holden B. Validation of the flush method as an alternative to basal or reflex tear collection. Curr Eye Res. 2011;36:198–207. doi: 10.3109/02713683.2010.542867. [DOI] [PubMed] [Google Scholar]
- 26.Sack RA, Tan KO, Tan A. Diurnal tear cycle: evidence for a nocturnal inflammatory constitutive tear fluid. Invest Ophthalmol Vis Sci. 1992;33:626–640. [PubMed] [Google Scholar]
- 27.Vijay AK, Sankaridurg P, Zhu H, Willcox MD. Guinea pig models of acute keratitis responses. Cornea. 2009;28:1153–1159. doi: 10.1097/ICO.0b013e3181a87a0b. [DOI] [PubMed] [Google Scholar]
- 28.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Overhage J, Bains M, Brazas MD, Hancock REW. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J Bacteriol. 2008;190:2671–2679. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 31.Cole N, Willcox MD, Fleiszig SM, Stapleton F, Bao B, Tout S, Husband A. Different strains of Pseudomonas aeruginosa isolated from ocular infections or inflammation display distinct corneal pathologies in an animal model. Curr Eye Res. 1998;17:730–735. [PubMed] [Google Scholar]