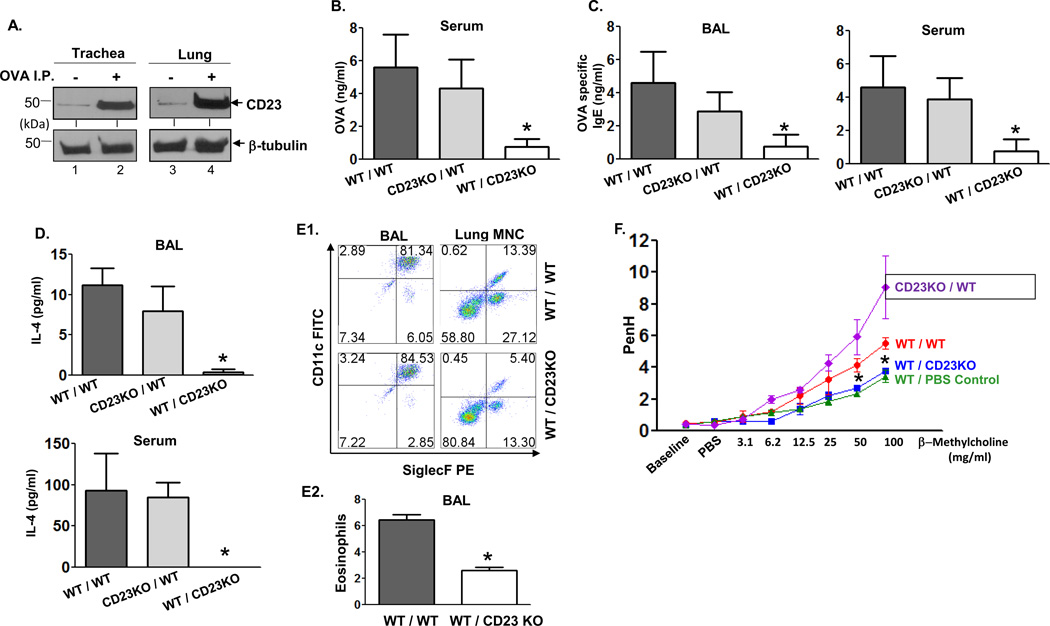
Fig 4. Epithelial CD23 facilitates airway inflammation after OVA sensitization and challenge in chimeric mice.
A. Mice were sensitized (i.p.) with OVA or left untreated. Lung and trachea epithelial cells were isolated and cell lysates (50 µg) were subjected to 12 % SDS-PAGE gel electrophoresis under reducing conditions. Proteins were transferred onto nitrocellulose membranes and blotted with rat-anti mouse CD23 mAb (B3B4) followed by HRP-conjugated rabbit anti-rat IgG Ab. Proteins were visualized using ECL. Arrows indicate mouse CD23 and β-tubulin.
B–D. WT/WT, CD23KO/WT, WT/CD23KO chimeric mice were i.p. sensitized with 100 µg OVA plus 4 mg alum at day 0 and subsequently injected (i.p.) with 100 µg OVA on day 7 and 14. Mice were challenged on day 21 with nebulized 1 % OVA for 30 min. 5 h after OVA challenge, sera were collected and OVA quantitated by ELISA (B). 24 h after OVA challenge, BAL fluid and sera were collected and OVA-specific IgE (C) and IL-4 (D) concentrations assessed by ELISA. *P<0.05.
E. Flow cytometric analysis of eosinophils in BAL fluid. Chimeric mice were sensitized with OVA and received a single aerosol OVA challenge. Total leukocytes obtained from BAL fluid were gated on CD45+ CD11bhi/int and mononuclear cells obtained from lung were gated on CD45+ CD11bhi cells and further analyzed for CD11c and Siglec-F expression (E1). The mean percentage of eosinophils in BAL fluid from three different experiments was calculated (E2). *P<0.05.
F. Airway responsiveness to methacholine (MCh) in chimeric and control mice. WT/WT, CD23KO/WT, and WT/CD23KO chimeric mice were sensitized (i.p.) with OVA and received a single aerosol challenge with OVA while control mice were sensitized and received a single aerosol challenge with PBS. Airway resistance was measured 24 h after challenge. First, the baseline and resistance against PBS challenge were measured followed by generation of a dose-response curve against an increasing concentration of nebulized MCh (3–100 mg/ml). *P<0.05.