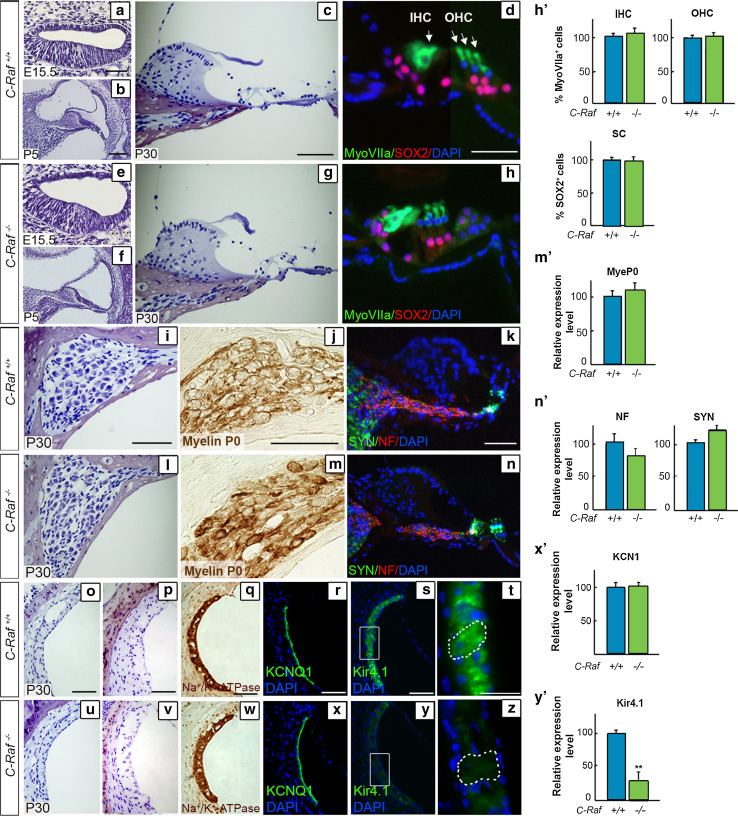
Fig. 3.
Characterization of C-Raf null mice cochlear cytoarchitecture and cell-type markers showing reduced levels of the Kir4.1 potassium channel, (a–c, e–g). Representative microphotographs of cross sections from wild-type and null mouse cochleae at E15.5, P5 and P30 (d, h, h′). The degree of Myosin VIIa (green) immunostaining/μm2 (outer and inner hair cells, OHC and IHC, respectively) and of SOX2 (red) (supporting cells, SC) was quantified in the organ of Corti (i, l). Neuronal distribution in the spiral ganglion was similar in P30 wild-type and null mice. Quantification of the intensity/μm2 of myelin P0 (j, m, m′), neurofilament (NF, red) and synaptophysin (SYN, green) (k, n, n′) staining showed no differences between genotypes. Representative microphotographs of paraffin-embedded (o, u) and semi-thin celloidin-embedded (p, v) cross sections of the stria vascularis from P30 wild-type and null mice (q, r, w, x, x′). Immunostaining of Na+/K+-ATPase and KCNQ1 channels in sections of wild-type mice and null mouse cochlea (s, t, y, z, y′). The Kir4.1 potassium channel expression level, also known as KCNJ10, was reduced in intermediate cells of null mice. Quantification of the immunofluorescence signal/μm2 was done using ImageJ software. Data were obtained from 4 to 12 sections of at least three mice from each genotype and plotted in bar graphs as the mean ± SEM relative to wild-type values. The significance of the differences was evaluated using Student’s t test: **P < 0.005 versus wild type. Scale bars 10 μm (d, h, t, z); 25 μm (a–c, e–g, i–n, o–s, u–y)