Abstract
Progressive multifocal leukoencephalopathy (PML) is a fatal demyelinating disease caused by neurotropic polyomavirus, JC (JCV), a virus that causes lytic infection of CNS glial cells. After primary infection, JCV is controlled by the immune system but virus persists asymptomatically. Rarely when immune function is impaired, it can re-emerge to cause PML. The mechanisms of JCV persistence and reactivation are not well understood but our earlier work implicated epigenetic control by protein acetylation since histone deactelylase inhibitors such as trichostatin A (TSA) strongly stimulate JCV transcription. Since both TNF-α and TSA activate JCV transcription via the same unique NF-κB site in the JCV control region, we investigated a role for acetylation of NF-κB in JCV regulation. A site-directed mutagenesis strategy was employed targeting the known lysine acetylation sites of NF-κB p65: K218, K221 and K310. We individually mutated each lysine to arginine, which cannot be acetylated and retains a positive charge like lysine. K218R and K221R impaired transactivation of JCV early promoter transcription either alone or combined with TSA treatment or coexpression of acetyltransferase transcriptional coactivator p300 but K310R was largely without effect. Mutation of lysine to glutamine gives mutants with a negative charge like acetyl-lysine. However, K218Q and K221Q showed impaired activity and only K310Q showed enhanced transactivation. NF-κB acetylation can regulate several aspects of the process of activation including complex formation with IκB, translocation to the nucleus and DNA binding and transcriptional activation. Cell fractionation studies revealed that the mutants had no defect in translocation to the nucleus whereas gel shift studies revealed reduced binding to the JCV NF-κB site. Thus acetylation regulates NF-κB p65 activity toward JCV at the level of p65 binding to the JCV control region and activation of JCV transcription.
Keywords: JC Virus, Progressive multifocal leukoencephalopathy, Viral persistence, Viral reactivation
INTRODUCTION
The human polyomavirus JC (JCV) is the etiological agent of the demyelinating CNS disease, progressive multifocal leukoencephalopathy. PML involves the cytolytic destruction of oligodendrocytes by JCV replication in the white matter of the brain leading to expanding lesions of myelin loss that can be debilitating and often lethal (Berger, 2011). As well as myelin loss, PML lesions are characterized histopathologically by oligodendrocytes with viral nuclear inclusion bodies and bizarre astrocytes, which are also productively infected by JCV (Khalili et al. 2008). PML is a very rare disease occurring almost exclusively in the context of severe impairment of the immune system notably HIV-1/AIDS where it remains a complication even after introduction of combination antiretroviral therapy (Tavazzi et al. 2012). Another important predisposing condition for PML is exposure to therapeutic immunomodulatory monoclonal antibodies such as natalizumab (Berger et al. 2010) and since these drugs are widely used to treat diseases such as rheumatoid arthritis, multiple sclerosis and Crohn’s disease, this is of considerable public health significance. Serological studies show that infection by JCV is a common event and usually occurs during childhood, after which it is clear that the virus is efficiently contained in most people (White and Khalili, 2011). It is only on rare occasions that JCV can reactivate and replicate in the brain leading to PML and our research has focused on the molecular mechanisms whereby this occurs early in the pathogenesis of the disease.
JCV has a circular double-stranded DNA genome of ~5 Kb in size and organized into an early region encoding large T-antigen and small t-antigen and a late region encoding the capsid proteins and agnoprotein (Padgett et al. 1971; DeCaprio et al. 2013). A third region lying between them, the noncoding regulatory region (NCCR), controls transcription and contains the viral origin of DNA replication. The NCCR contains binding sites for a number of transcription factors, which regulate early and late transcription (White et al. 2009) including a unique NF-κB site found in the highly conserved early proximal part of the NCCR that confers strong transcriptional activation by NF-κB (Ranganathan and Khalili, 1993; Romagnoli et al. 2009) and is also regulated by interplay of NF-κB p65 with C/EBPβ (Romagnoli et al. 2009), NFAT4 (Wollebo et al. 2012) and Rad51 (White et al. 2014). Furthermore, JCV transcription is also stimulated via the NF-κB binding site in response to treatment of cells with proinflammatory cytokines such as TNF-α (Wollebo et al. 2011).
Recently, we also found that JCV transcription is strongly activated by the histone deacetylation inhibitors (HDACi) trichostatin A (TSA) and sodium butyrate while the DNA methylation inhibitor 5-azacytidine had no effect suggesting that viral gene expression is regulated epigenetically by events that involve protein acetylation (Wollebo et al. 2013). In this regard, latent JCV is similar to HIV-1 where latent virus is reactivated by HDACi and this has been suggested as a means to reactivate latent HIV reservoirs for eradication (Shirakawa et al. 2013). Since most people contain reservoirs of persistent asymptomatic JCV, the potential of JCV reactivation by HDACi is an important consideration.
Interestingly, the regulation of JCV by HDACi appears to be associated with the NF-κB binding site since mutations in the JCV NCCR that prevented NF-κB p65 binding blocked the effect of TSA as did siRNA to NF-κB p65. Gel shift assays with a double-stranded oligonucleotide corresponding to the NF-κB site showed that ectopic p65 expression, TNF-α stimulation or TSA treatment each caused a gel shift of the same mobility that was further shifted by antibody to p65 (Wollebo et al. 2013). Thus, JCV is regulated epigenetically by protein acetylation events, which involve the NF-κB binding site in the JCV NCCR.
Acetylation of histones is catalyzed by histone acetyltransferase enzymes (HAT) and acetyl group removal by histone deacetylases (HDAC). Both HAT and HDAC act not only on histones but also on nonhistone proteins including certain transcription factors such a NF-κB p65 (Quivy and Van Lint, 2004; Calao et al. 2008). Thus we reasoned that it was possible that the effect of HDACs on JCV transcription that we observed might be mediated by acetylation of NF-κB p65. In vivo acetylation of NF-κB p65 principally targets lysines 218, 221 and 310. Findings from investigations of these acetylation sites highlight how site-specific acetylation of p65 differentially regulates different biological activities of the NF-κB complex (Chen et al. 2002). To investigate a role for NF-κB p65 in JCV transcriptional regulation, we used a base substitution mutagenesis strategy directed at lysine residue acetylation sites to investigate their functional importance. Lysine (K) can be mutated to arginine (R) to give a site that cannot be acetylated and so always retains its positive charge or to glutamine (Q) to mimic acetylated lysine with a negative charge. We have now assessed the effects of the p65 lysine mutants on regulation of JCV. Our data indicate that acetylation of NF-κB p65 regulates its activity toward JCV at the level of p65 binding to the JCV control region and activation of JCV transcription.
METHODS
Cell culture and plasmids
Culture of the human TC620 oligodendroglioma cell line was performed as we have previously described (Wollebo et al. 2011). The reporter construct JCVE-LUC contains the JCV promoter from the Mad-1 strain linked to the luciferase gene in the early orientation (Wollebo et al. 2011). The expression plasmid pCMV-p65(wt) was described previously (Romagnoli et al. 2009) and expression plasmids for the p65 acetylation site Lys to Arg mutants (p65K218R, p65K221R and p65K310R) were purchased from Addgene (Cambridge, MA) and recloned into pCDNA6B (Life Technologies, Inc., Grand Island, NY) to give pCMVK218R, pCMVK221R and pCMVK310R respectively. Expression plasmids for the p65 acetylation site Lys to Gln mutants (p65K218Q, p65K221Q and p65K310Q) were made by site directed mutagenesis of pCMV-p65(wt) using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent, Wilmington DE) as we have previously described (Romagnoli et al. 2009).
Antibodies
The following antibodies were used for Western blot: Rabbit polyclonal anti-p65 (c-20, sc-372, Santa Cruz Biotechnology Inc., Santa Cruz, CA), anti-α-tubulin clone B512 from Sigma-Aldrich (St. Louis, MO) and anti-Lamin A/C from Cell Signaling (Danvers, MA).
Western blots
Western blot assays were performed as previously described (White et al. 2006). Briefly, 50 μg of protein was resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with primary antibody (1/1000 dilution) and secondary antibody (1/10000 dilution). Bound antibody was detected with an ECL detection kit (Amersham, Arlington Heights, IL).
Transient transfection and reporter assays
Co-transfection of reporter plasmids and expression plasmids were performed using Lipofectamine 2000 (Life Technologies Inc., Carlsbad CA) as we have previously described (Romagnoli et al. 2009; Wollebo et al. 2011). Briefly, TC620 cells were transfected with reporter constructs alone or in combination with expression plasmid(s) for 48 h prior to harvesting. The total amount of transfected DNA was normalized with empty vector DNA. Treatment with trichostatin A (TSA, 300 ng/ml) was performed for 24 h prior to harvesting. Assay for luciferase was performed as previously described (Romagnoli et al. 2009; Wollebo et al. 2011).
Cell fractionation
TC620 cells were transfected with each of the wild-type and mutant p65 expression plasmids for 48 h, treated with and without 10 ng/ml TNF-α for 30 min and then harvested for nuclear and cytoplasmic fractions using the NE-PER nuclear and cytoplasmic extraction kit according to the Manufacturer’s instructions (Thermo Scientific Pierce, Rockford IL; Cat. # PI-78835).
Gel shift
TC620 cells were transfected with each of the wild-type and mutant p65 expression plasmids for 48 h, treated overnight with 250 nM TSA and then harvested and nuclear extracts prepared. Ten micrograms of nuclear proteins were incubated with 50,000 cpm of a γ-32P-labeled, double-stranded oligodeoxyribonucleotide probe as previously described (Romagnoli et al. 2009). The probe used in these gel shift experiments corresponded to the JCV NF-κB site: 5′-aaaacaagggaatttccctggcctc-3′ (nts 5052–5078, Mad-1 JCV, GenBANK # NC_001699).
RESULTS
Mutation of p65 Lys to Arg at K218 and K221 but not K310 inhibits transactivation of the JCV early promoter both in the presence and absence of TSA
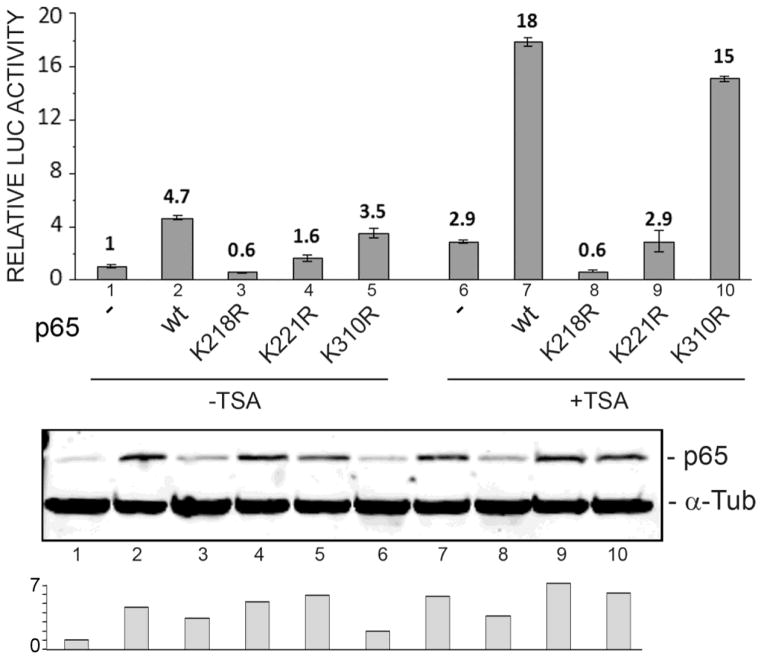
In vivo acetylation of NF-κB p65 principally targets lysines 218, 221 and 310 and has site-specific differential regulatory biological effects on the activities of the NF-κB complex (Chen et al. 2002). We used a base substitution mutagenesis strategy directed at the lysine residue acetylation sites to investigate their functional importance. TC620 cells were transfected with JCV early promoter reporter plasmid and expression plasmid for wild-type, K218R, K221R or K310R NF-κB p65 with and without TSA treatment. As shown in Fig. 1, wild-type NF-κB p65 stimulated early promoter transcription about four- to five-fold (lane 2) similar to our previous reports (Romagnoli et al. 2009; Wollebo et al. 2012, 2013, 2014). This was reduced to 0.6- and 1.6-fold in the K218R and K221R mutants respectively (lanes 3 and 4). However, the K310R mutant was only slightly less effective (3.5-fold, lane 5) than wild-type p65. Similarly, treatment with TSA stimulated early promoter transcription about 3-fold (lane 6) and was highly cooperative with expression of wild-type p65 (18-fold, lane 7) as we have previously reported (Wollebo et al. 2013). The K218R and K221R mutants were greatly muted in their response to TSA (0.6- and 2.9-fold, lanes 8 and 9 respectively) while the K310R mutant was only slightly less effective in stimulating early promoter transcription with TSA (15-fold, lane 10) than the wild-type (18-fold, lane 7). There was a slight variability in the levels of expression of the different forms of p65 in the transfected cells (lanes 2–5, 7–10) but this cannot account for the differences in promoter activities relative to wild-type seen with the K218R and K221R. We conclude the adjacent acetylation sites at positions 218 and 221 in p65, but not the site at 310, are important for mediating the stimulatory effects of p65 and TSA on JCV early promoter transcription.
Figure 1. Effect of p65 Lys to Arg mutants on transactivation of the JCV early promoter.
TC620 cells were transfected with JCVE-Luc reporter plasmid and NF-κB p65 expression plasmid (wild-type, K218, K221R or K310R) and then untreated or treated with 300 ng/ml TSA 24 h later. After a further 24 h, cells were harvested and assayed for luciferase activity as described in Methods. Activities were normalized to untreated controls. The bar represents one standard deviation. The histogram at the bottom shows quantification of the Western. The experiment was performed at least twice.
Coexpression of p300 and p65 mutants showed strongly reduced transactivation of the JCV early promoter for K218R and K221R mutants and a slight reduction for K310R
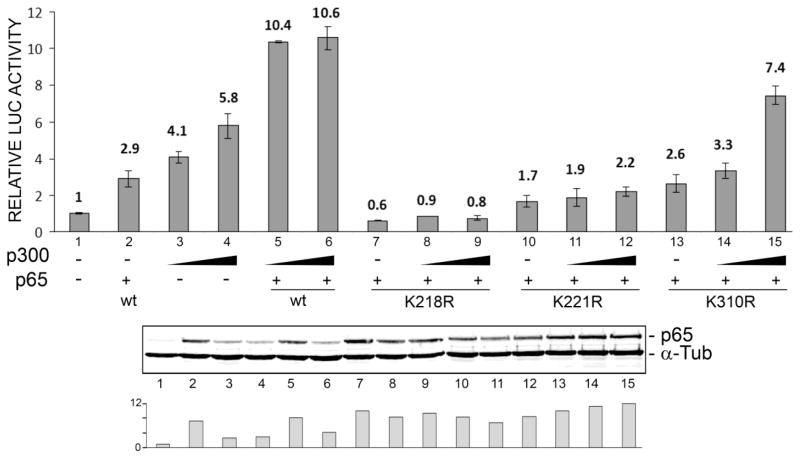
The histone acetyltransferase and transcriptional co-activator p300 directly binds to p65 and potentiates p65-activated transcription (Gerristen et al. 1997). This is associated with the acetylation of p65 principally targeting K218, K221 and K310 (Chen et al. 2002). We investigated a role for p300 and acetylation by examining the effect of co-expression of p300 and each of the p65 acetylation site Lys to Arg mutants. As shown in Figure 2, expression of p300 alone stimulated JCV early promoter transcription up to ~6-fold (lane 4) while expression of p300 in the presence of wild-type p65 stimulated up to ~11-fold (lane 6). For the p65K218R mutant, stimulation by p300 was abolished (lanes 7–9) and for the p65K221R mutant, it was reduced to ~2-fold (lanes 10–12). The stimulation by p65K310R was reduced but to a much lesser extent (up to 7.4-fold, lane 15). Thus K218 and K221 acetylations are important for p65 stimulation of JCV early promoter transcription in conjunction with p300.
Figure 2. Effect of coexpression of p300 and p65 Lys to Arg mutants on transactivation of the JCV early promoter.
TC620 cells were transfected with the reporter plasmid JCVE-LUC and NF-KB p65 expression plasmid (wild-type, K218R, K221R or K310R) together with expression plasmid for p300 (0, 0.25 and 0.5 μg), harvested and assayed for luciferase activity. Activities were normalized to untreated controls. The bar represents one standard deviation. The histogram at the bottom shows quantification of the Western. The experiment was performed at least twice.
Mutation of p65 Lys to Gln at K218 and K221 inhibits transactivation of the JCV early promoter both in the presence and absence of TSA whereas Lys to Gln mutation at K310 enhances
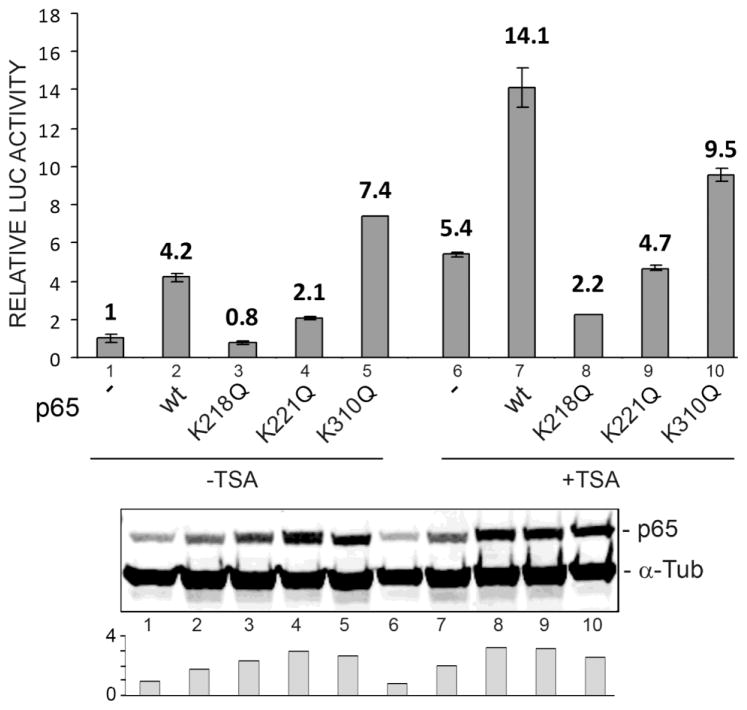
Mutation of key lysine residues at K218 or K221 to arginine, which cannot be acetylated and retains its positive charge, has a negative effect on the transactivation by p65 of the JCV early promoter. We reasoned that mutation to glulamine (Gln, Q) to mimic negatively charged acetylated lysine, might have a positive effect on transactivation. However this was not the case. As shown in Figure 3, the K218Q and K221Q mutants negatively impacted transactivation with a reduction to 0.8- and 2.1-fold respectively (lanes 3 and 4). Only the K310Q mutant was more effective (7.4-fold, lane 5) than wild-type p65 and this was only the case in the absence of TSA. We conclude that an unacetylatable mutation or an acetylation-mimicking mutation at either K218 or K221 is inactivating and hence reversible acetylation events at positions 218 and 221 in p65 are important for mediating the stimulatory effects of p65 and TSA on JCV early promoter transcription and as will be discussed below.
Figure 3. Effect of p65 Lys to Gln mutants on transactivation of the JCV early promoter.
TC620 cells were transfected with JCVE-Luc reporter plasmid and NF-κB p65 expression plasmid (wild-type, K218Q, K221Q or K310Q) and then untreated or treated with 300 ng/ml TSA 24 h later. After a further 24 h, cells were harvested and assayed for luciferase activity as described in Methods. Activities were normalized to untreated controls. The bar represents one standard deviation. The histogram at the bottom shows quantification of the Western. The experiment was performed at least twice.
Coexpression of p300 and p65 mutants showed strongly reduced transactivation of the JCV early promoter for K218Q and K221Q mutants and a slight reduction for K310Q
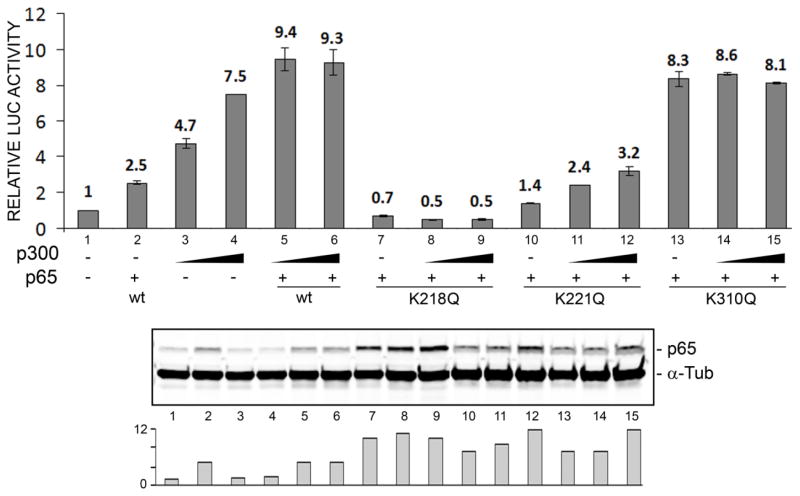
We further investigated the role of p300 and acetylation by examining the effect of co-expression of p300 and each of the p65 acetylation site Lys to Gln mutants. As shown in Figure 4, expression of p300 in the presence of the p65K218Q mutant, the stimulation seen for wild-type p65 was abolished (lanes 7–9) and for the p65K221Q mutant, it was much reduced (lanes 10–12). However, stimulation by p65K310Q was as about as strong (8–9-fold, lanes 13–15) as wild-type p65. These data are in agreement with the TSA data in Fig. 3, i.e., reversible acetylation by p300 occurring at positions 218 and 221 in p65 is important for mediating the stimulatory effects of p65.
Figure 4. Effect of coexpression of p300 and p65 Lys to Gln mutants on transactivation of the JCV early promoter.
TC620 cells were transfected with the reporter plasmid JCVE-LUC and NF-κB p65 expression plasmid (wild-type, K218Q, K221Q or K310Q) together with expression plasmid for p300 (0, 0.25 and 0.5 μg), harvested and assayed for luciferase activity. Activities were normalized to untreated controls. The bar represents one standard deviation. The histogram at the bottom shows quantification of the Western. The experiment was performed at least twice.
Expression of p65 wild-type and mutants showed similar subcellular distribution
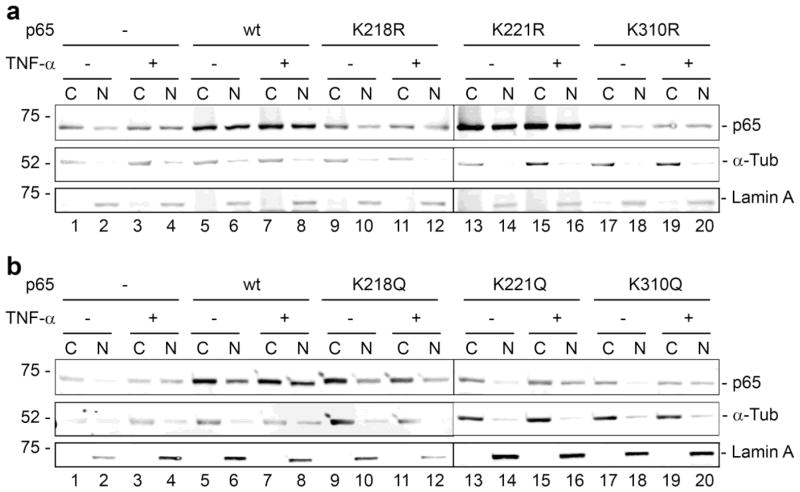
The NF-κB pathway involves mobilization of NF-κB from a complex in the cytoplasm with IκB and translocation into the nucleus. To investigate if altered translocation was involved in the loss of transactivation ability of the acetylation site mutants of p65, cell fractionation experiments were performed with TC620 cells that were untreated or treated with TNF-α, which causes the dissociation of NF-κB and I-κB in the cytoplasm and the translocation of NF-κB to the nucleus (Hoffmann and Baltimore, 2006). TC620 cells were transfected with NF-κB p65 expression plasmids (wild-type, K218R, K221R, K310R, K218Q, K221Q or K310Q), treated with and without TNF-α and then harvested for nuclear and cytoplasmic fractions. As expected, endogenous p65 moved from cytoplasm to nucleus after TNF-α addition (Fig. 5a and 5b, lanes 1–4). Ectopically expressed wild-type p65 was present at high levels in both the cytoplasm and nucleus with or without TNF-α (Fig. 5a and 5b, lanes 5–8). For the mutants, no significant decrease in nuclear p65 was detected indicating that a defect migration of p65 is likely not responsible for the transcriptional differences.
Figure 5. Effect of p65 Lys to Arg and Lys to Gln mutants on subcellular localization of p65.
TC620 cells were transfected with NF-κB p65 expression plasmid (wild-type, K218R, K221R, K310R, K218Q, K221Q or K310Q), treated with and without TNF-α and then harvested for nuclear and cytoplasmic fractions as described in Materials and Methods. Western blots are performed for each fraction for p65 and purity and equal loading was assessed by Western for cytoplasmic α-tubulin and nuclear lamin. a. K to R mutants. b. K to Q mutants. The line between lanes 12 and 13 in each panel indicates that samples 1–12 and 13–20 were run in parallel on separate gels. The experiment was performed twice.
NF-κB p65 Lys to Arg and Lys to Gln mutants show reduced binding of p65 to the JCV NF-κB binding site
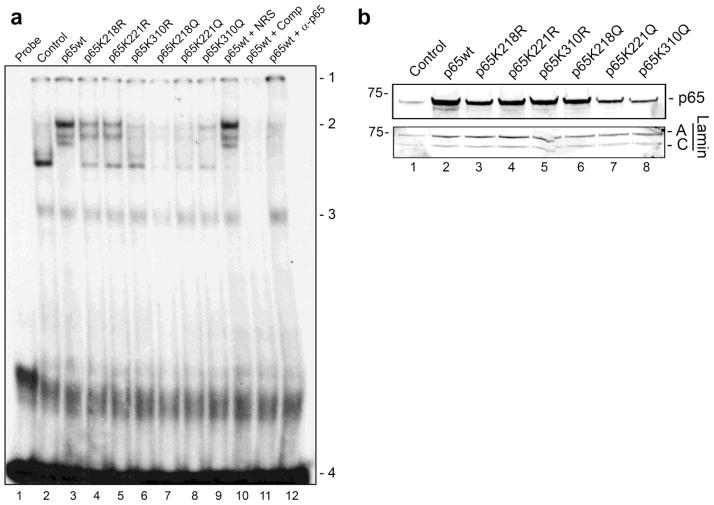
Gel shifts experiments were performed using nuclear extracts from TC620 cells transfected with p65, wild-type and mutants and an oligonucleotide probe corresponding to the JCV NF-κB site, which showed a gel shift upon expression of p65 (position 2). Wild-type p65 gave a robust gel shift (lane 2). All mutants showed reduced binding compared to the wild-type. Of note, p65K218Q gel shift was barely detectable (lane 6), which correlates with its much reduced ability to transactivate the JCVE promoter (Figs. 3 and 4). Specificity of the gel shift was demonstrated using unlabeled probe DNA (lane 11), which removed the band and the appearance of a supershift band (position 1) with antibody specific to p65 (lane 12) but not nonimmune rabbit serum (lane 10).
DISCUSSION
Our previous studies had indicated that JCV transcription is strongly activated by the histone deacetylation inhibitors trichostatin A (TSA) and sodium butyrate suggesting that viral gene expression is regulated epigenetically by events that involve protein acetylation (Wollebo et al. 2013). Mutations in the JCV NCCR that prevent NF-κB p65 binding block the effect of TSA as does siRNA to NF-κB p65 suggesting that NF-κB has a role in mediating the effect of TSA on JCV transcription. Furthermore, gel shift assays with an NF-κB site probe showed that ectopic NF-κB p65 expression and TNF-α stimulation each caused a gel shift that was further shifted by antibody to p65 and that this shift was also elicited by TSA treatment (Wollebo et al. 2013). Thus, we hypothesized that acetylation of NF-κB p65 is involved in transactivation of JCV transcription. To test this hypothesis, we created mutations at three lysine residues in the p65 protein, K218, 221 and 310, which are known to be the principal acetylation sites of NF-κB p65 in vivo (Chen et al. 2002). Separate mutation of each of these sites to arginine, which cannot be acetylated, revealed that K218 and K221 mutations are inhibitory and hence these two adjacent lysine residues are involved in p65 activation of the JCV early promoter. Interestingly, although we initially thought that mutation of these lysines to glutamine, which mimics the negative charge of acetylated lysine, might enhance transactivation, we found that K218Q and K221Q mutations are also inhibitory. Thus, it is likely that transitions between acetylated/deacetylated states are involved in the regulation of different aspects of activation of NF-κB, which is complex and involves multiple levels of control including binding and dissociation from other proteins, e.g., IκB, subcellular translocation, binding to specific DNA sequences, phosphorylation, etc. Acetylation of p65 has been reported to affect a number of different NF-κB functions including transcriptional activation, DNA-binding affinity and complex formation with IκBα in the cytoplasm (Chen et al. 2002; Quivy and Van Lint, 2004; Schmitz et al. 2004; Calao et al. 2008).
Investigation of cytoplasmic sequestration and nuclear translocation of the NF-κB p65 mutants showed there was no alteration, i.e., our data indicate that translocation of p65 between cytoplasm and nucleus is not responsible for the differences observed in the ability of the p65 acetylation site mutants to transactivate the JCV promoter. However, the mutants showed reduced binding compared to the JCV NF-κB DNA sequence when compared to wild-type p65.
In conclusion, our evidence suggests NF-κB is regulated by acetylation at K218 and K221, which determines its capacity to activate JCV gene expression and provides an epigenetic mechanism for regulating JCV persistence and activation. This is another complexity to those we have reported, such as NF-κB binding to C/EBPβ (Romagnoli et al. 2009), NFAT4 (Wollebo et al. 2012) and Rad51 (White et al. 2014), NF-κB induction in response to treatment of cells with proinflammatory cytokines such as TNF-α (Wollebo et al. 2011) and the induction of Rad51 by JCV infection, which acts through NF-κB/Rad51 binding to stimulate JCV transcription by a positive feedback mechanism (Wollebo et al. 2014). Together, these findings indicate NF-κB is a crucial control nexus where diverse signals converge and lead to a switch between asymptomatic JCV persistence and initiation of viral reactivation. Experimental and mathematical modeling of NF-κB signaling have suggested that there exists a threshold level of input allowing a switch-like response (Shinohara et al. 2014) and such a mechanism may operate to trigger the change between silent JCV in asymptomatic persistence and actively replicating JCV upon initiation of PML pathogenesis. Exploring such a model provides a promising future direction for research.
Figure 6. Effect of p65 Lys to Arg and Lys to Gln mutants on binding of p65 to the JCV NF-κB binding site.
a. TC620 cells were transfected with NF-κB p65 expression plasmid (wild-type, K218R, K221R, K310R, K218Q, K221Q or K310Q) for 48 h and nuclear extracts harvested. An oligonucleotide corresponding to the JCV NCCR KB element was used to perform EMSA using nuclear extracts from untreated cells (Control) or cells transfected with expression vector for p65, untreated or treated with TSA overnight as described in Methods. For supershifts, antibody to p65 (α-p65) or nonimmune rabbit serum (NRS) was added as indicated. Competitor–excess unlabelled probe was added. Unbound probe (loaded alone in lane 1) runs near the bottom of the gel (position 4). b. Western blot for the expression of p65 is shown. The experiment was performed twice.
Acknowledgments
We thank past and present members of the Center for Neurovirology for their insightful discussion and sharing of ideas and reagents. Anna Bellizzi was partially supported by postdoctoral fellowship “Teresa Ariaudo 2013” from Institute Pasteur Cenci-Bolognetti Foundation. We thank Valeria Pietropaolo for her discussion and input into this study. This study utilized services offered by Temple University School of Medicine Comprehensive NeuroAIDS Center supported by NIH grant P30MH092177. This work was supported by grant R01 AI077460 awarded by the NIH to MKW.
Footnotes
CONFLICT OF INTEREST
The authors Hassen S. Wollebo, Anna Bellizzi, Dominique H. Cossari, Mahmut Safak, Kamel Khalili and Martyn K. White declare that they have no conflicts of interest.
References
- Berger JR. Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf. 2010;33:969–983. doi: 10.2165/11537510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Berger JR. The clinical features of PML. Cleve Clin J Med. 2011;78(Suppl 2):S8–12. doi: 10.3949/ccjm.78.s2.03. [DOI] [PubMed] [Google Scholar]
- Calao M, Burny A, Quivy V, Dekoninck A, Van Lint C. A pervasive role of histone acetyltransferases and deacetylases in an NF-kappaB-signaling code. Trends Biochem Sci. 2008;33:339–349. doi: 10.1016/j.tibs.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio JA, Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Lippincott, Williams & Wilkins; Philadelphia: 2013. pp. 6133–1661. [Google Scholar]
- Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Khalili K, Safak M, Del Valle L, White MK. JC virus molecular biology and the human demyelinating disease, progressive multifocal leukoencephalopathy. In: Shoshkes Reiss C, editor. Neurotropic virus infections. Cambridge University Press; Cambridge, UK: 2008. pp. 190–211. [Google Scholar]
- Padgett BL, Zu Rhein GM, Walker DL, Echroade R, Dessel B. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet. 1971;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Quivy V, Van Lint C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem Pharmacol. 2004;68:1221–1229. doi: 10.1016/j.bcp.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Ranganathan PN, Khalili K. The transcriptional enhancer element, kappa B, regulates promoter activity of the human neurotropic virus, JCV, in cells derived from the CNS. Nucleic Acids Res. 1993;21:1959–1964. doi: 10.1093/nar/21.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, Del Valle L, Safak M, Khalili K, White MK. Modulation of JC virus transcription by C/EBPβ. Virus Res. 2009;146:97–106. doi: 10.1016/j.virusres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz ML, Mattioli I, Buss H, Kracht M. NF-kappaB: a multifaceted transcription factor regulated at several levels. Chembiochem. 2004;5:1348–1358. doi: 10.1002/cbic.200400144. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Behar M, Inoue K, Hiroshima M, Yasuda T, Nagashima T, Kimura S, Sanjo H, Maeda S, Yumoto N, Ki S, Akira S, Sako Y, Hoffmann A, Kurosaki T, Okada-Hatakeyama M. Positive feedback within a kinase signaling complex functions as a switch mechanism for NF-κB activation. Science. 2014;344:760–764. doi: 10.1126/science.1250020. [DOI] [PubMed] [Google Scholar]
- Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 2013;21:277–285. doi: 10.1016/j.tim.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi E, White MK, Khalili K. Molecular insights into polyomavirus JC and clinical updates on progressive multifocal leukoencephalopathy. Rev Med Virol. 2012;22:18–32. doi: 10.1002/rmv.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy–revisited. J Infect Dis. 2011;203:578–586. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Skowronska A, Gordon J, Del Valle L, Deshmane SL, Giordano A, Khalili K. Analysis of a mutant p53 protein arising in a medulloblastoma from a mouse transgenic for the JC virus early region. Anticancer Res. 2006;26:4079–4092. [PubMed] [Google Scholar]
- White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol. 2009;83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Kaminski R, Khalili K, Wollebo HS. Rad51 activates polyomavirus JC early transcription. PLoS One. 2014;9:e110122. doi: 10.1371/journal.pone.0110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollebo HS, Del Valle L, Safak M, Khalili K, White MK. Role for tumor necrosis factor-alpha in JC virus reactivation and progressive multifocal leukoencephalopathy. J Neuroimmunol. 2011;233:46–53. doi: 10.1016/j.jneuroim.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollebo HS, Melis S, Khalili K, Safak M, White MK. Cooperative Roles of NF-κB and NFAT4 in polyomavirus JC regulation at the KB control element. Virology. 2012;432:146–154. doi: 10.1016/j.virol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollebo HS, Woldemichaele B, Khalili K, Safak M, White MK. Epigenetic regulation of polyomavirus JC. Virol J. 2013;10:264. doi: 10.1186/1743-422X-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]