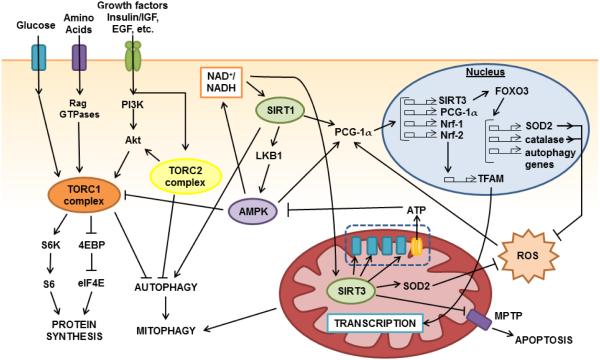
Figure 2. Mammalian energy-sensing pathways implicated in dietary interventions.
A complex crosstalk exists between the energy-sensing pathways in mammals. The complexes of the mTOR pathway, TORC1 and TORC2 are activated by signals of high nutrient availability. TORC1 is stimulated by the presence of amino acids, growth factors including insulin, ATP, and glycolysis metabolites. TORC2 is also activated by growth factor signaling including insulin and EGF and further activates TORC1 through Akt. Together, TORC1 and TORC2 stimulate cell growth and proliferation through the inhibition of cell maintenance pathways such as autophagy, and the stimulation of global protein synthesis. Conversely, AMPK inhibits TORC1 and is activated by a low energy status in the cell. AMPK also contributes activate the sirtuins, including SIRT1 and SIRT3, and is in turn further activated by SIRT1. SIRT1 leads to the activation of PGC-1α. PGC-1α activation induces the transcription of many important genes involved in mitochondrial biogenesis and regulation, including Nrf-1, and Nrf-2, which induce expression of TFAM, and also further transcriptionally activates itself and SIRT3. Meanwhile, SIRT3 activates FoxO3, which induces the expression of key ROS antioxidants such as SOD2 and catalase as well as key genes involved in autophagy. SIRT1 further activates autophagic machinery by deacetylation of key atophagic components. Activation of autophagy can consequently activate mitophagy, leading to selective clearance of damaged or unfit mitochondria. In the mitochondria, SIRT3 also deacetylates many proteins including complexes of the electron transport chain, MPTP component cyclohpilin D, and SOD2, resulting in greater respiratory efficiency, apoptotic resistance, and ROS protection. Arrows indicate activation. T bars indicate suppression.