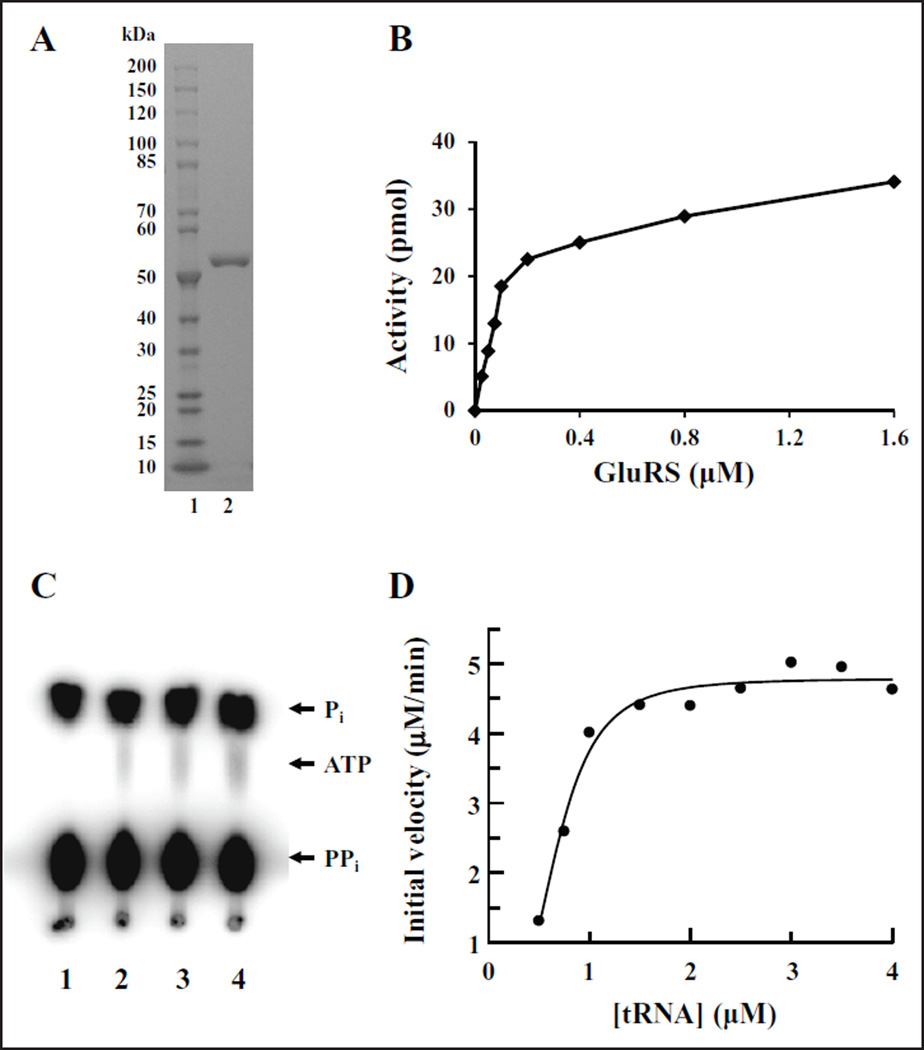
Figure 2.
Purification and characterization of P. aeruginosa GluRS. (A) One microgram of purified P. aeruginosa GluRS was analyzed on a 4–20% SDS-PAGE gel and the protein bands were visualized by staining with Coomassie blue. Lane 1 contains 10–200 kD protein standard; lane 2 contains P. aeruginosa GluRS. (B) P. aeruginosa GluRS was titrated into the aminoacylation assay as described in the Materials and Methods section at concentrations between 0.025 and 1.6 µM. Background activity was minimal and was subtracted from values at all concentrations of GluRS. (C) ATP:PPi exchange reaction to determine the requirement for the cognate tRNA for formation of an aminoacyl-adenylate by P. aeruginosa GluRS. The reactions contained 0, 0.3, 0.6, and 1.2 µM tRNAGlu in lanes 1, 2, 3, and 4, respectively. (D) Determination of the kinetic parameters with respect to tRNAGlu for P. aeruginosa GluRS in the aminoacylation reaction. Initial velocities were determined and the data were fit to a Michaelis–Menten steady-state model using XLfit (IDBS) to determine KM and Vmax.