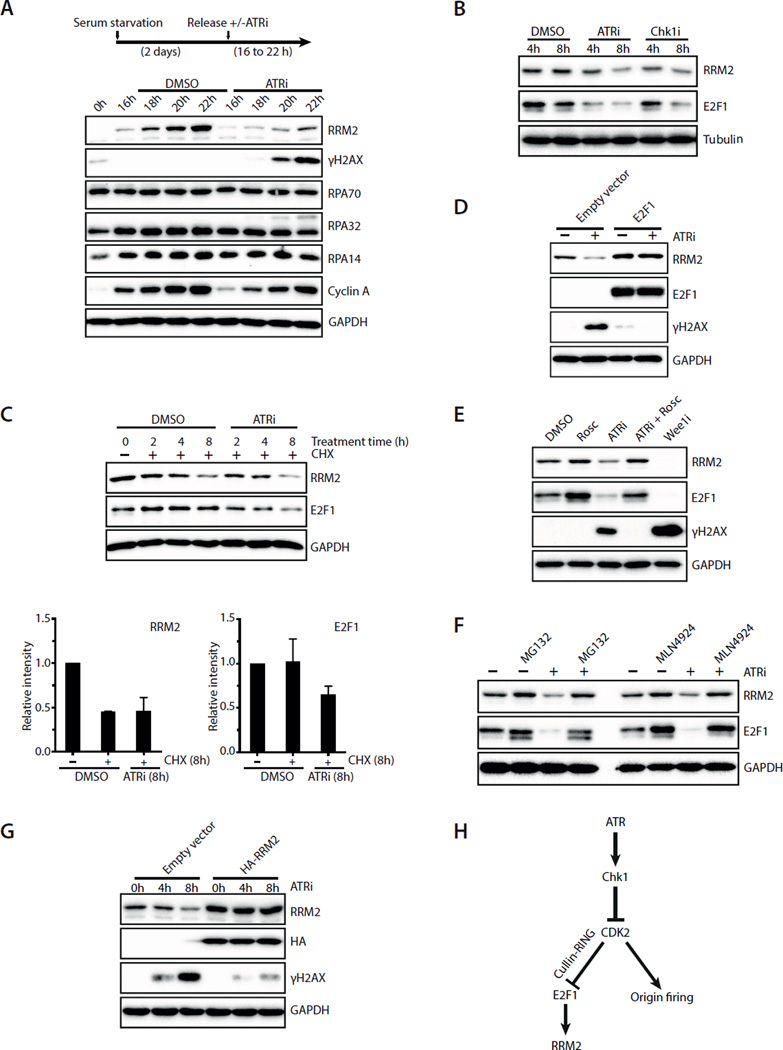
Fig. 3. ATRi suppresses DNA damage by promoting RRM2 accumulation and limiting origin firing.
A. T98G cells were synchronously released from G0 in the presence or absence of ATRi (10 μM VE-821). Levels of RRM2, γH2AX, RPA70, RPA32, RPA14 and Cyclin A were analyzed during the time course. B. Asynchronously growing U2OS cells were treated with ATRi (10 μM VE-821) or Chk1i (2 μM MK-8776). Levels of RRM2 and E2F1 were analyzed at the indicated times. C. Levels of RRM2 and E2F1 were analyzed in U2OS cells treated with DMSO or ATRi in the presence of cycloheximide (CHX). Relative levels of RRM2 and E2F1 were quantified from 3 blots (n=3). Error bars: S.D. D. U2OS cells transfected with empty vector or E2F1-expressing plasmids were treated with ATRi for 8 h. Levels of RRM2, E2F1, and γH2AX were analyzed. E. U2OS cells were treated with the indicated inhibitors for 8 h. Levels of RRM2, E2F1, and γH2AX were analyzed. F. U2OS cells were treated with DMSO or ATRi for 8 h in the presence or absence of MG132 or MLN4924. G. U2OS cells infected with HA-RRM2-expressing retrovirus or control virus were treated with ATRi for 8 h. Levels of RRM2 and γH2AX were analyzed at the indicated times. H. A model in which ATR coordinates RRM2 accumulation and origin firing in early S phase. See also Fig. S3.