Abstract
Background & Aims
Polycystic liver diseases (PLDs) are genetic disorders characterized by progressive biliary cystogenesis. Current therapies show short-term and/or modest beneficial effects. Cystic cholangiocytes hyperproliferate as a consequence of diminished intracellular calcium levels ([Ca2+]i). Here, the therapeutic value of ursodeoxycholic acid (UDCA) was investigated.
Methods
Effect of UDCA was examined in vitro and in polycystic (PCK) rats. Hepatic cystogenesis and fibrosis, and the bile acid (BA) content were evaluated in liver, bile, serum and kidneys by HPLC-MS/MS.
Results
Chronic treatment of PCK rats with UDCA inhibits hepatic cystogenesis and fibrosis, and improves their motor behaviour. As compared to wild-type animals, PCK rats show increased BA concentration ([BA]) in liver, similar hepatic Cyp7a1 mRNA levels, and diminished [BA] in bile. Likewise, [BA] is increased in cystic fluid of PLD patients compared to their matched serum levels. In PCK rats, UDCA decreases the intrahepatic accumulation of cytotoxic BA, normalizes their diminished [BA] in bile, increases the BA secretion in bile and diminishes the increased [BA] in kidneys. In vitro, UDCA inhibits the hyperproliferation of polycystic human cholangiocytes via a PI3K/AKT/MEK/ERK1/2-dependent mechanism without affecting apoptosis. Finally, the presence of glycodeoxycholic acid promotes the proliferation of polycystic human cholangiocytes, which is inhibited by both UDCA and tauro-UDCA.
Conclusions
UDCA was able to halt the liver disease of a rat model of PLD through inhibiting cystic cholangiocyte hyperproliferation and decreasing the level of cytotoxic BA species in the liver, which suggests the use of UDCA as a potential therapeutic tool for PLD patients.
Keywords: ursodeoxycholic acid (UDCA), cystogenesis, cholangiocyte, intracellular calcium, therapy
Polycystic liver diseases (PLDs) are a group of genetic disorders characterized by bile-duct dilation and/or development of biliary cysts, which progressively grow and are the main cause of morbidity [1]. The large volume of hepatic cysts may cause different symptoms and complications such as severe hepatomegaly, abdominal distension with local pressure, back pain, hypertension, gastro-esophageal reflux, dyspnea, as well as bleeding, infection and/or rupture of the cysts [1]. PLDs are inherited in dominant or recessive form and developed alone or in association with polycystic kidney diseases (PKDs). Autosomal dominant polycystic liver disease (ADPLD) develops cysts preferentially in the liver, whereas the kidney is also markedly affected in autosomal dominant (ADPKD) and recessive (ARPKD) forms of PKDs [1]. Local surgery of symptomatic cysts is commonly employed and partially prevents and/or ameliorates the disease progression. However, this therapy show short-term and/or modest beneficial effects, liver transplantation being the only curative option [1].
Current research is focused on elucidating the molecular mechanisms involved in the pathogenesis of PLDs with the aim of identifying new potential targets for pharmacological therapy [2]. Novel evidence suggests that PLDs share several pathological mechanisms that may provide a key for treatment [2]. In this regard, cAMP-mediated hyperproliferation of cystic cholangiocytes is considered a central event in PLDs [2]. Different experimental and clinical studies have previously demonstrated the potential therapeutic value of somatostatin analogues in partially reducing the increased cAMP concentration [cAMP]i and the concomitant cAMP-mediated hyperproliferation in cystic cholangiocytes [3–10], indicating that drugs specifically directed to inhibit this pathway could have key therapeutic value. In this regard, we have previously reported that cholangiocytes from PCK rats, an animal model of ARPKD, are characterized by increased [cAMP]i and diminished intracellular calcium concentration [Ca2+]i [11]. Restoration of [Ca2+]i with a calcium ionophore inhibited both basal and cAMP/PKA/MEK/ERK1/2-stimulated proliferation of PCK rat cholangiocytes via activation of the PI3K/AKT pathway [11]. These data indicate that normalization of [Ca2+]i in cystic cholangiocytes may have translational impact. Different molecules and mechanisms are able to upregulate the [Ca2+]i in cholangiocytes. In particular, ursodeoxycholic acid (UDCA; 3α, 7β-dihydroxy-5β-cholanoic acid) is a minor endogenous hydrophilic bile acid (BA) used for the treatment of several cholestatic disorders [12, 13]. Experimental evidence suggests that UDCA stimulates the hepatobiliary secretion of bicarbonate and protects cholangiocytes against the cytotoxicity of hydrophobic BAs, and that these effects are mediated in part by increasing the [Ca2+]i [12, 13]. In an experimental model of obstructive cholestasis, such as bile-duct ligation (BDL) in rats, cholangiocytes hyper-proliferate as a consequence of increased [cAMP]i and decreased [Ca2+]i resulting in bile-duct proliferation. Interestingly, treatment of this animal model with UDCA for 1 week inhibits the aforementioned cholangiocyte hyperproliferation by normalizing the [Ca2+]i [14, 15].
In the present study, we explored the potential therapeutic value of UDCA in inhibiting the hepatic cystogenesis of experimental models of PLD and the molecular mechanisms of action.
Materials and Methods
Treatment of PCK Rats with UDCA
The PCK rat (Charles River Laboratory) is a well characterized animal model of ARPKD that presents a spontaneous mutation in the PKHD1 orthologous gene [16, 17]. Hepatic cystogenesis and fibrosis, as well as serum biochemical markers were analyzed in non-treated wild-type (n=12) and PCK (n=10) rats, as well as in PCK rats (n=10) orally-treated with UDCA (25 mg/kg/day) for 5 months. The details are described in Supplementary data.
Determination of Messenger RNA Expression
Total RNA was obtained from rat liver tissue with TriReagent (Sigma). Detection and quantification of messenger RNAs (mRNAs) were performed via reverse-transcription polymerase chain reaction (RT-PCR) and real-time quantification (qPCR) as we previously described [18] (cf. Supplementary Table 1 for specific primers). The Gapdh gene expression was employed as a normalizing control.
Western Blot Analysis
Changes in protein expression and/or phosphorylation were detected through immunoblotting using cell extracts or PCK rat liver tissue as detailed in Supplementary data.
Bile Acid Measurement
BAs were analyzed in total liver, bile collected before the sacrifice, peripheral blood and total kidney of rats by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) after C18-based extraction as we previously reported [19, 20]. Similarly, BA concentration ([BA]) was determined in cystic fluid of patients with PLD and compared with their paired samples of peripheral blood. All human procedures were approved by the Medical Ethical Committee of the Radboudumc, Nijmegen, The Netherlands.
Animal Motor Behavior
The degree of motor activity in PCK rats was analyzed using the Open field test [21]. One day prior to sacrifice, both untreated and UDCA-treated PCK rats were individually placed in the center of a quadrangular box (511 cm × 511 cm) and the free trajectory was monitored for 5 minutes using an infrared x-ray camera. The distance and the average speed were measured. Each animal performed one trial.
Isolation and Culture of Normal and Polycystic Human Cholangiocytes
Normal and polycystic human cholangiocytes were isolated and characterized as we previously described [18].
Measurement of Intracellular Ca2+ Level in Normal and Polycystic Human Cholangiocytes in Culture
Intracellular calcium level was determined as we previously described [11]. Briefly, normal and polycystic human cholangiocyte cultures were loaded with 4 µM Fura-2/AM (Biotium) for 30 min in HCO3− -Ringer´s solution (with 2 mM CaCl2) at 37°C and washed twice. Then, the fluorescence intensities (dual excitation at 340/380 nm and emission at 510 nm) were measured overtime in the presence or absence of 100 µM UDCA using an inverted Leica DMIRB fluorescent microscope equipped with a software to acquire and analyze the images.
Proliferation Assays in Normal and Polycystic Human Cholangiocytes in Culture
Proliferation of both normal and polycystic human cholangiocytes was assessed in the presence or absence of different doses of UDCA, TUDCA (tauroursodeoxycholic acid) or GDCA (glycodeoxycholic acid) (ie. 100, 200 and/or 500 µM) and/or inhibitors (MEK inhibitor U0126: 15 µM; Calbiochem) in quiescent medium (DMEM-Ham´s-F12 with 3% fetal bovine serum and 1% penicillin/streptomycin) for 48 hours at 37°C using the CellTrace™ CFSE Cell Proliferation Kit (Invitrogen) for flow cytometry (FC 500 MCL System, Beckman Coulter).
Evaluation of Apoptosis in Polycystic Human Cholangiocytes in Culture
The rate of apoptosis was determined in both normal and polycystic human cholangiocytes using the FITC Annexin V Apoptosis Detection Kit II (BD Pharmingen) for flow cytometry. Briefly, normal and polycystic human cholangiocytes were cultured in the presence or absence of different doses of UDCA (ie. 100, 200 and 500 µM) in quiescent medium for 48 hours at 37°C. Then, cells were incubated with the aforementioned kit following the manufacturer guidelines and apoptotic rates were measured in a FC 500 MCL fluorometer.
Statistical Analysis
Data are shown as mean ± SEM. Once normality was assessed with D’Agostino-Pearson or Shapiro-Wilks tests, we used the Student t-test for statistical comparisons between two groups of normally distributed variables, and one-way analysis of variance (ANOVA) and subsequent Bonferroni post hoc test for comparisons between more than two groups. When non-parametric methods were required, Mann-Whitney tests were used. Analyses were carried out with the GraphPad Prism 5 statistical software.
Results
Beneficial effect of UDCA treatment in PCK rats
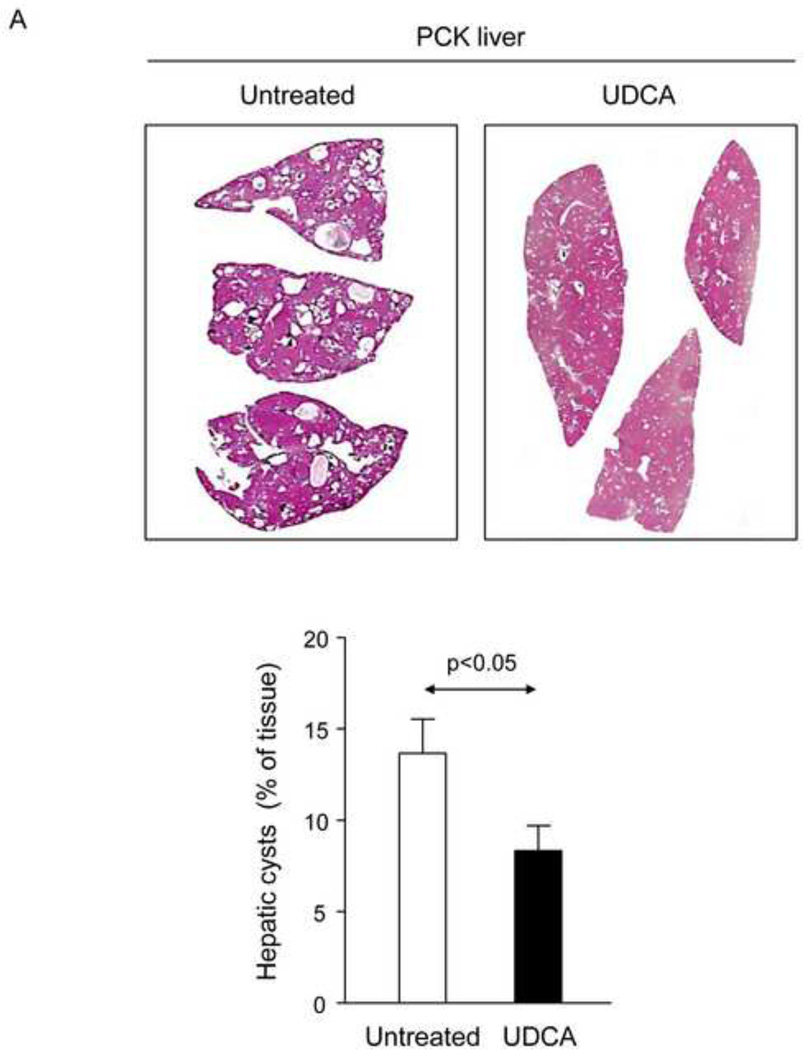
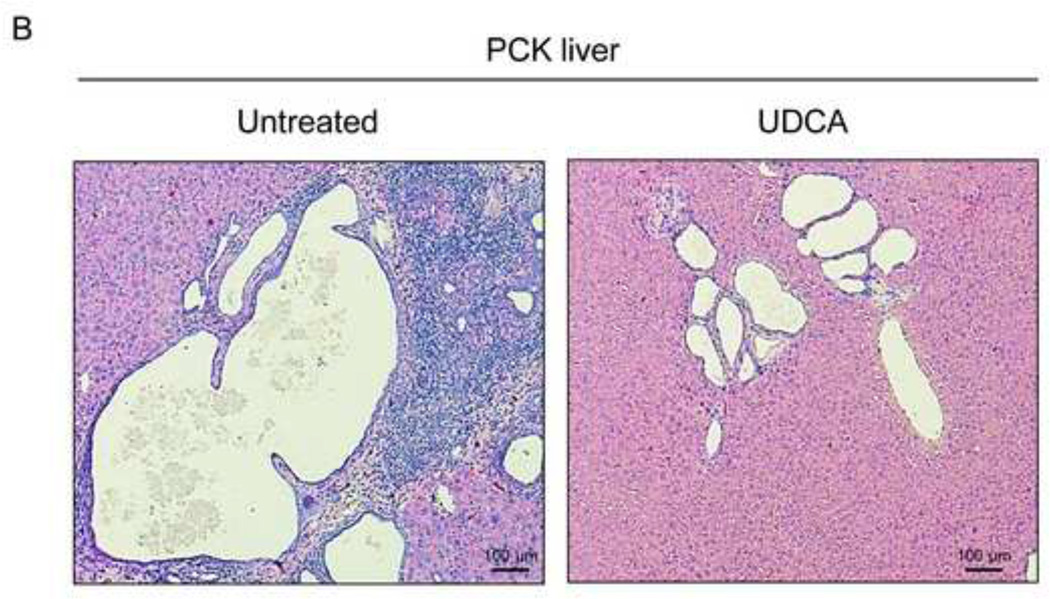
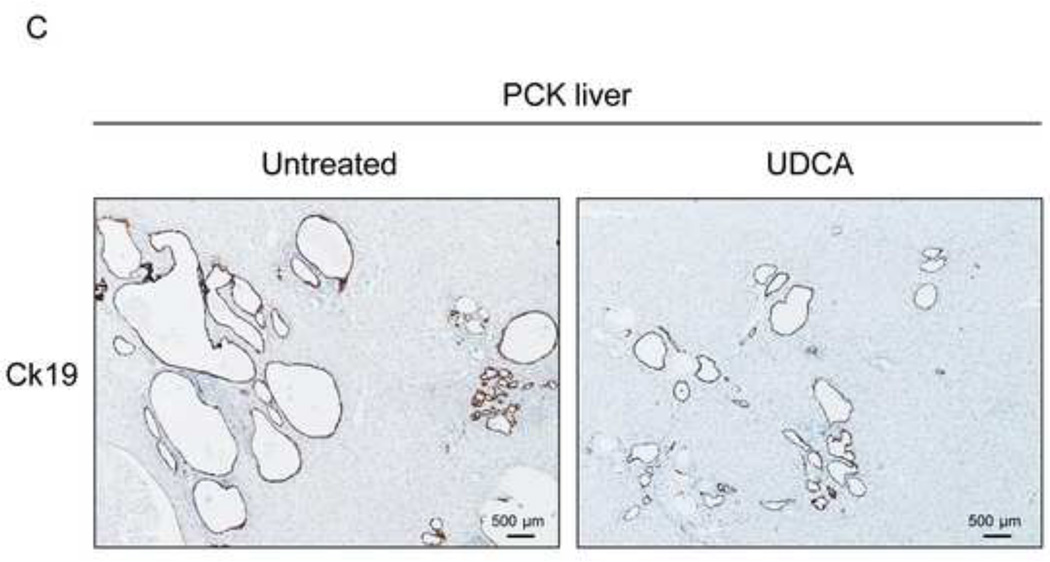
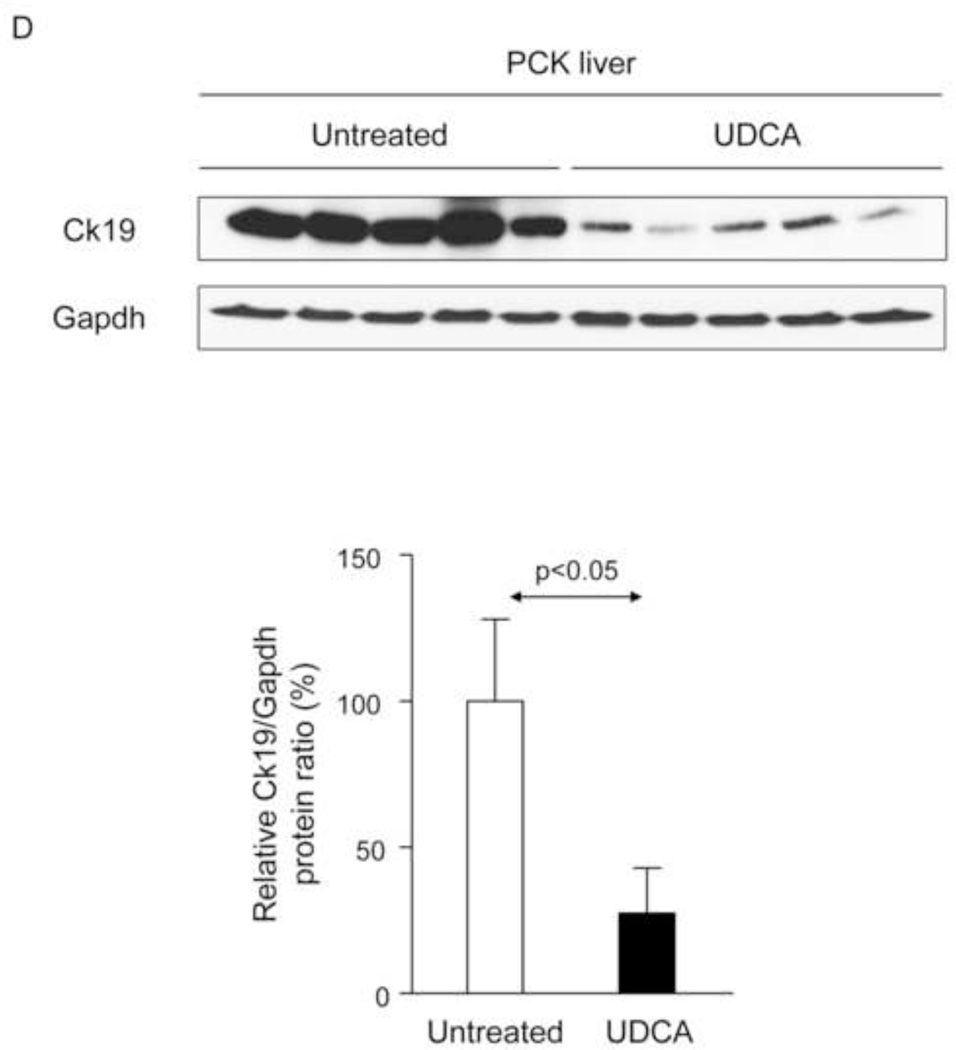
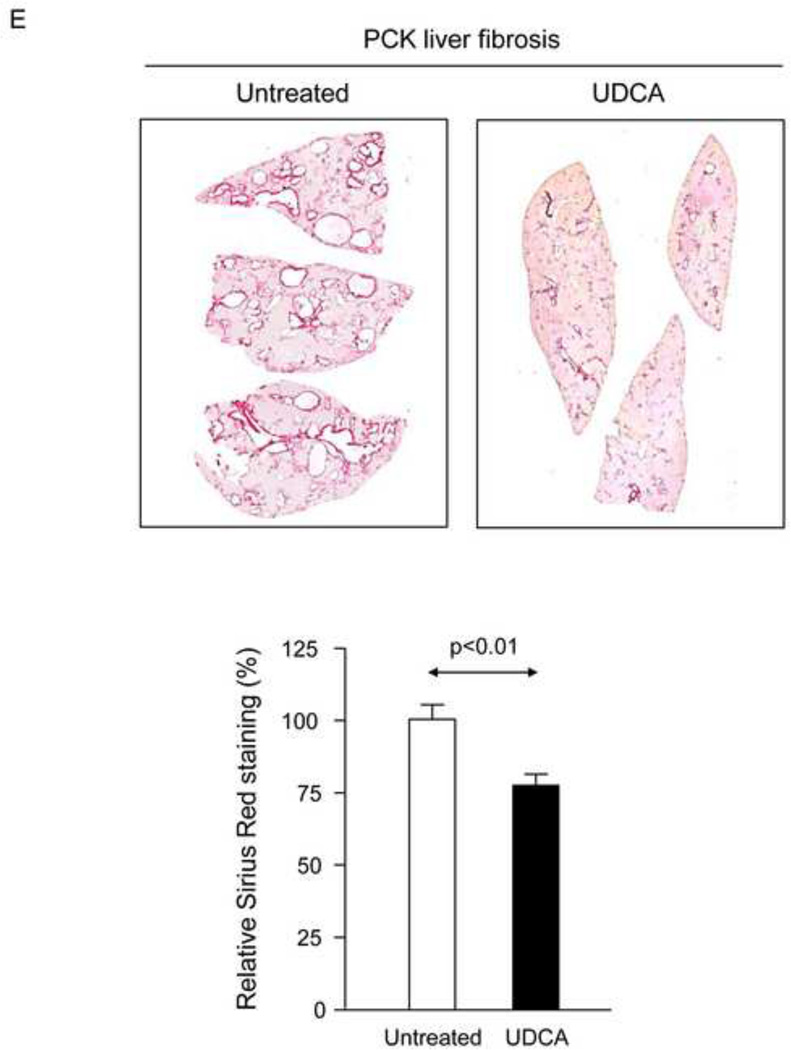
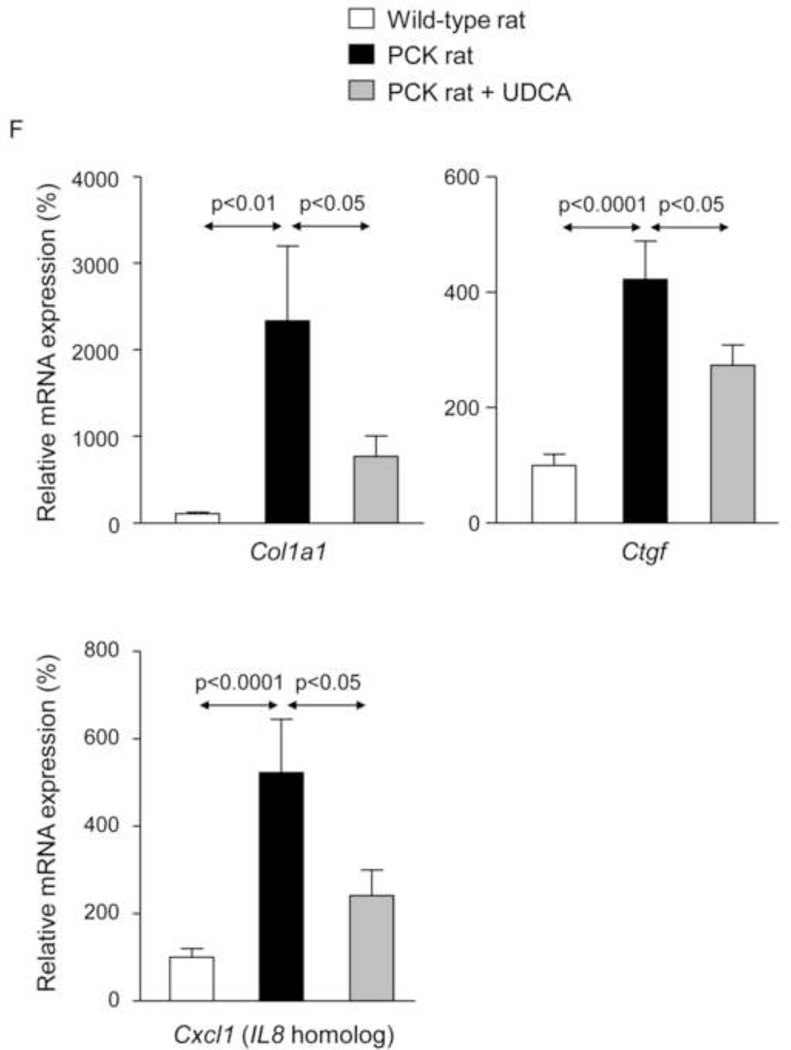
To evaluate the potential therapeutic value of UDCA for the treatment of PLDs, it was administered to 8-week-old PCK rats for 5 months. Treatment of PCK rats with UDCA decreased hepatic cystogenesis (Figs. 1A,B); this event was associated with decreased protein levels of the cholangiocyte-marker Ck19 in the liver (Figs. 1C,D). Moreover, PCK rats treated with UDCA showed lower levels of fibrosis −ie. less areas stained for collagen with Sirius Red (Fig. 1E) and decreased liver expression of both pro-fibrotic [collagen-1a1 (Col1a1) and connective tissue growth factor (Ctgf)] and pro-inflammatory [IL8-homolog (Cxcl1)] genes at mRNA level (Fig. 1F)−; on the other hand, no statistical differences in alpha-smooth muscle actin (α-SMA), transforming growth factor beta 1 (Tgfβ1) and interleukin 6 (IL6) were found at mRNA level (Suppl. Fig. 1). By contrast, UDCA treatment induced no significant change in the renal cystogenesis also developed in PCK rats (Suppl. Fig. 2).
Figure 1.
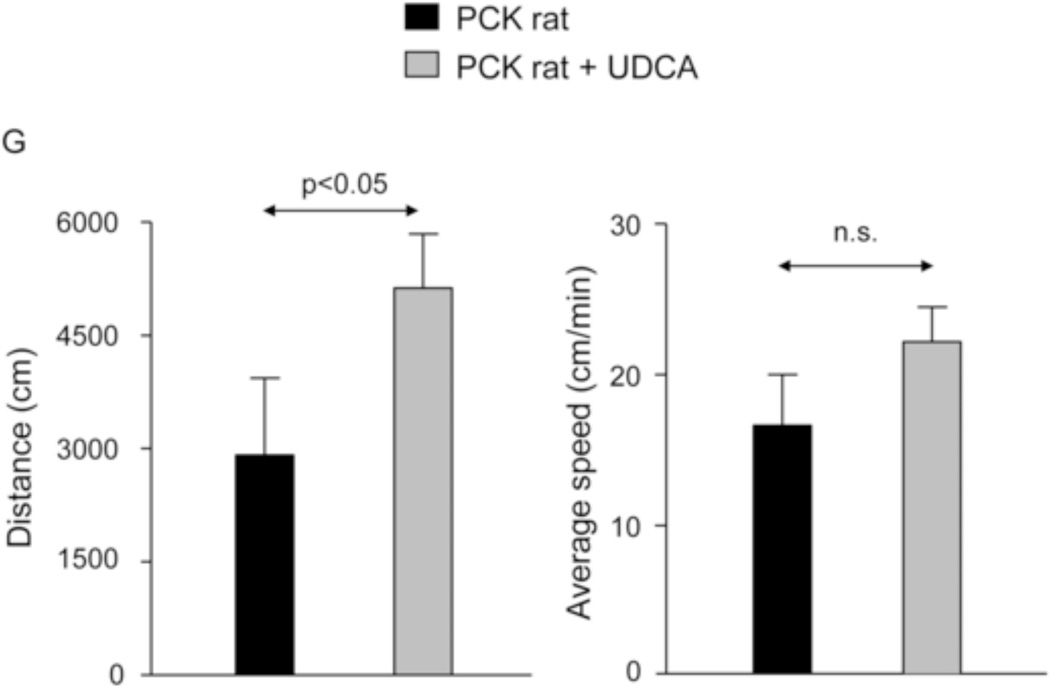
Treatment of PCK rats with UDCA halts hepatic cystogenesis and fibrosis, and improves their motor behaviour. (A and B) Representative images (hematoxylineosin staining; 5× magnification in Fig. 1A) and bar graph showing hepatic cysts in untreated and UDCA-treated PCK rats. (C and D) Hepatic expression of the cholangiocyte-marker Ck19 at protein level. Representative images of Ck19 immunohistochemistry (C) and western blots (D) of 5 representative untreated or UDCA-treated PCK rats. Bar graph shows Ck19 quantification (n=10 and n=9 in PCK and PCK+UDCA groups, respectively). (E) Representative images (Sirius Red staining; 5× magnification) and bar graph showing the hepatic collagen deposition in untreated and UDCA-treated PCK rats. (F) Expression levels (mRNA) of pro-fibrotic (Col1a1 and Ctgf) and pro-inflammatory (Cxcl1) genes in liver of wild-type and PCK (untreated and UDCA-treated) rats. (G) Open field test (distance and average speed) in untreated and UDCA-treated PCK rats.
N=12 in wild-type (untreated) and n=10 in PCK (untreated and UDCA-treated) groups unless specified.
As compared with wild-type animals, PCK rats showed increased liver weight, bile flow and serological alkaline phosphatase (ALP) levels, as well as decreased body weight and serological albumin and protein levels (Table 1). In PCK rats, UDCA administration was not accompanied by variation in body, liver or kidney weights, or changes in the serum levels of biochemical markers, such as ALP, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, urea, and albumin (Table 1). The serum levels of total protein were slightly decreased in PCK rats treated with UDCA compared with controls and the liver weight mean value had a tendency to decrease after UDCA treatment. On the other hand, UDCA treatment induced an almost statistically significant increase in bile flow of PCK rats compared to non-treated PCK controls (Table 1).
Table 1.
Characteristics of the animal groups at the moment of the sacrifice
| A | B | C | |||
|---|---|---|---|---|---|
| Parameters | Wild-type rats |
PCK Rats (non-treated) |
PCK Rats (UDCA-treated) |
P value (A vs B) |
P value (B vs C) |
| Body weight (g) | 675.9 ± 15.72 | 575.6 ± 12.42 | 573.8 ± 9.61 | .0001 * | .91 |
| Liver weight (g) | 20.69 ± 0.95 | 32.68 ± 3.99 | 26.2 ± 2.28 | .0045 * | .17 |
| Liver/body weight (%) | 3.09 ± 0.10 | 5.79 ± 0.82 | 4.598 ± 0.43 | .003 * | .21 |
| Kidney weight (g) | .............. | 2.71 ± 0.16 | 3.2 ± 0.64 | ..... | .46 |
| Kidney/body weight (%) | .............. | 0.9 ± 0.1 | 1.0 ± 0.1 | ..... | .37 |
| Bile flow (µL/min/g) | 0.337 ± 0.03 | 0.76 ± 0.13 | 1.033 ± 0.10 | .007 * | .13 |
| Alkaline phosphatase (U/L) | 89.83 ± 7.2 | 122.7 ± 13.96 | 117.9 ± 10.95 | .04 * | .79 |
| Aspartate aminotransferase (U/L) | 149.1 ± 32.64 | 176.7 ± 36.32 | 159.3 ± 17.85 | .57 | .67 |
| Alanine aminotransferase (U/L) | 59.0 ± 6.68 | 48.6 ± 3.89 | 53.7 ± 5.02 | .20 | .43 |
| Albumin (g/L) | 3.8 ± 0.05 | 2.04 ± 0.09 | 1.99 ± 0.06 | .0001 * | .66 |
| Protein total (g/dL) | 5.65 ± 0.08 | 4.13 ± 0.08 | 3.88 ± 0.05 | .0001 * | .02* |
| Bilirubin total (mg/dL) | .............. | 0.16 ± 0.0 | 0.17 ± 0.0 | ..... | .67 |
| Blood urea (mg/dL) | 33.6 ± 1.3 | 36.27 ± 2.5 | 33.6 ± 2.8 | .35 | .49 |
“Dot lines” indicate non-analyzed values
PCK rats tend to to be less mobile with the disease progression, possibly because of increasing discomfort and deterioration of their physical condition overtime. Since these animals were apparently less lethargic under UDCA treatment, their motor behaviour was evaluated using the Open field test, which is accepted as an indicator of the animal physical state [21]. The results indicated that PCK rats treated with UDCA for 5 months walked more distance than untreated PCK rats without affecting their average speed (Fig. 1G). These data suggest that UDCA treatment improves the physical state of PCK rats.
Effect of UDCA treatment on the bile acid levels in liver, bile, peripheral blood and kidney of PCK rats
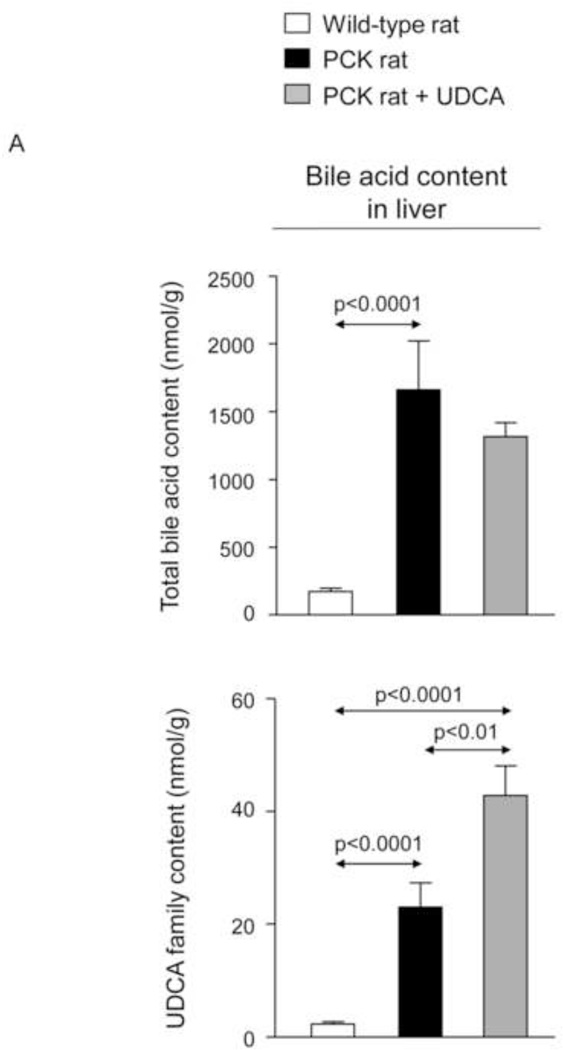
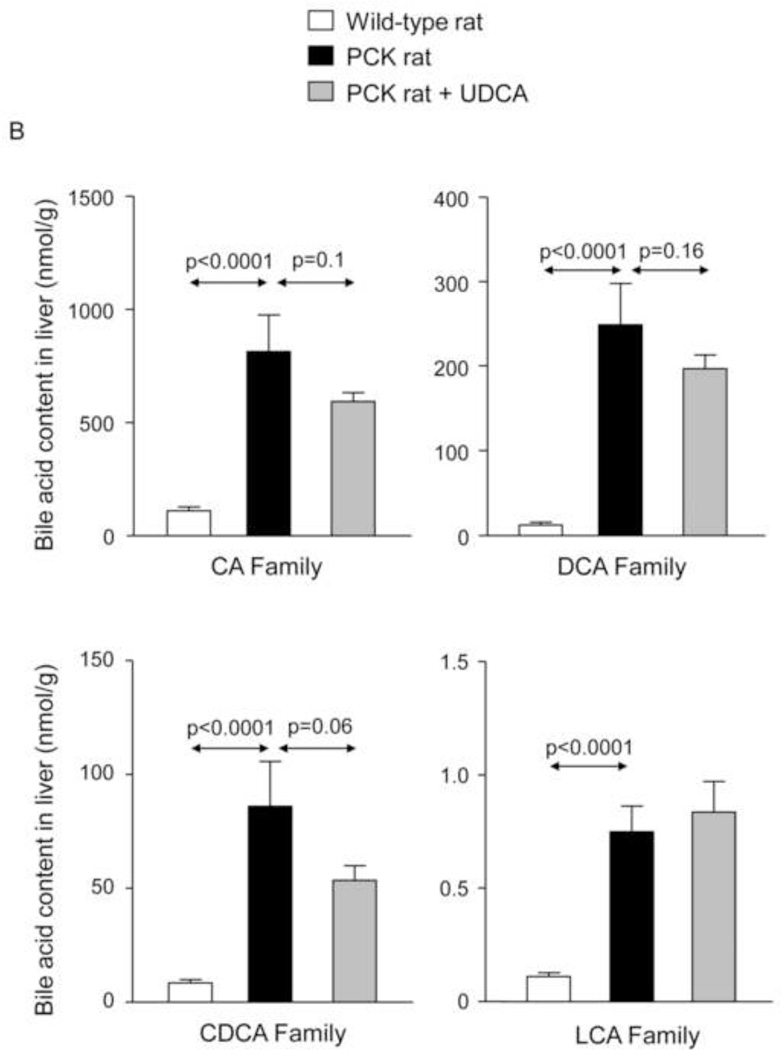
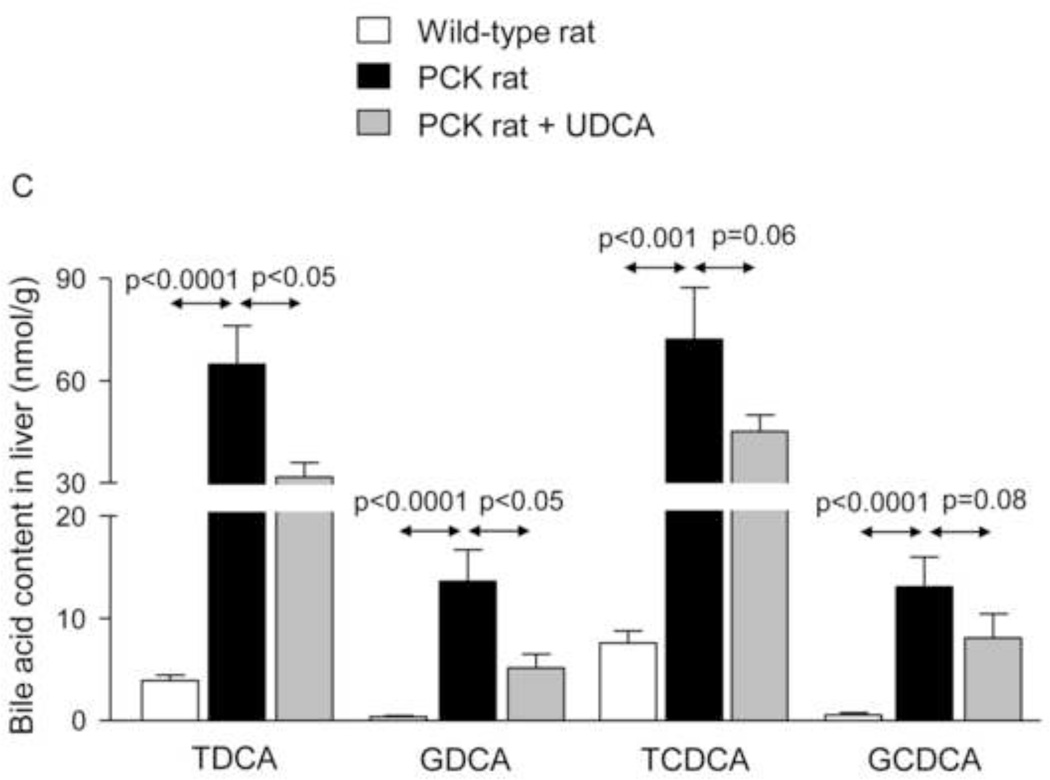
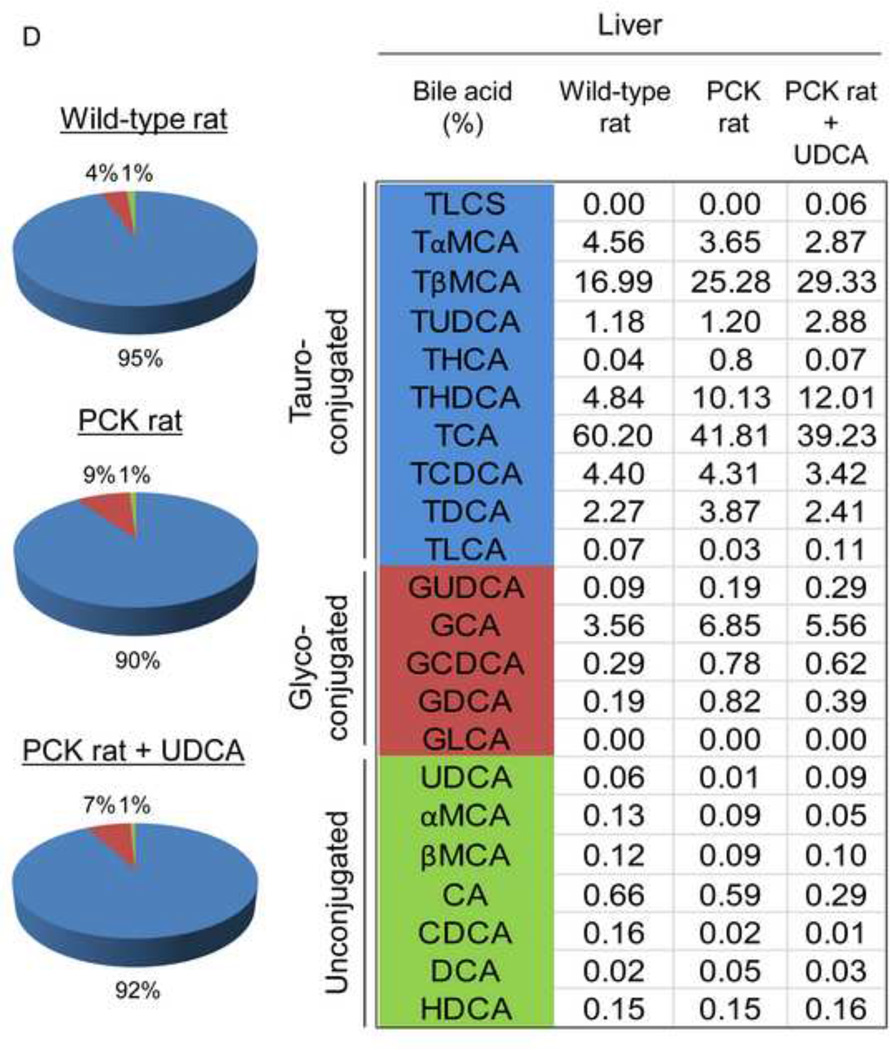
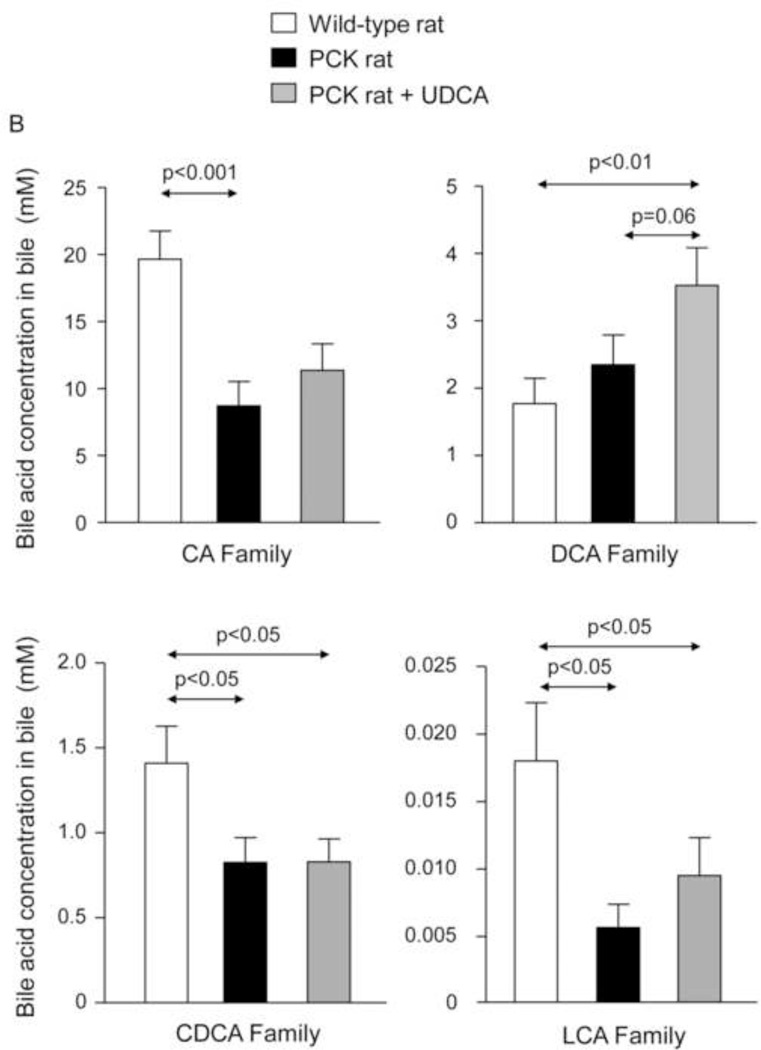
To investigate whether the beneficial effect induced by UDCA could be mediated in part by changes in the exposure of biliary tissue to the more toxic BA species, we analyzed the liver BA levels and composition of 7-month-old PCK (untreated and treated with UDCA for 5 months) and untreated wild-type rats. We found that PCK rats showed ~16-fold higher intrahepatic total [BA] compared to matched wild-type control rats (Fig. 2A). The levels of major BAs, both primary [ie. cholic acid (CA) and chenodeoxycholic acid (CDCA)] and secondary [ie. deoxycholic acid (DCA) and lithocholic acid (LCA)] species, were higher in the liver of PCK than wild-type rats (Fig. 2B). Moreover, the intrahepatic levels of the most abundant and toxic dihydroxylated BAs [ie. DCA and CDCA conjugated with either taurine (TDCA and TCDCA) or glycine (GDCA and GCDCA)] were markedly higher in PCK than in wild-type rats (Fig. 2C). Chronic treatment of PCK rats with UDCA did not alter the liver content of total BAs (Fig. 2A). However, UDCA treatment resulted in a trend to decreased intrahepatic concentrations of major BAs (Fig. 2B). When the more toxic species were analyzed separately, it was notable that UDCA treatment decreased the hepatic concentration of TDCA and GDCA and almost the concentration of TCDCA and GCDCA (Fig. 2C). However, the proportions of unconjugated and tauro- and glyco-conjugated BAs were similar in wild-type and PCK rats and was not substantially affected by UDCA treatment (Fig. 2D). In this regard, the percentage of UDCA family (unconjugated + glyco-conjugated + tauro-conjugated) in the hepatic BA pool was very low and similar in normal (1.32%) and untreated PCK (1.40%) rats, and increased (to 3.26%) after treatment of PCK rats with UDCA (Fig. 2D). Therefore, the increased UDCA concentration observed in the liver of untreated PCK rats compared to wild-type rats is linked to the general accumulation of BAs within the liver of PCK rats (Fig. 2A).
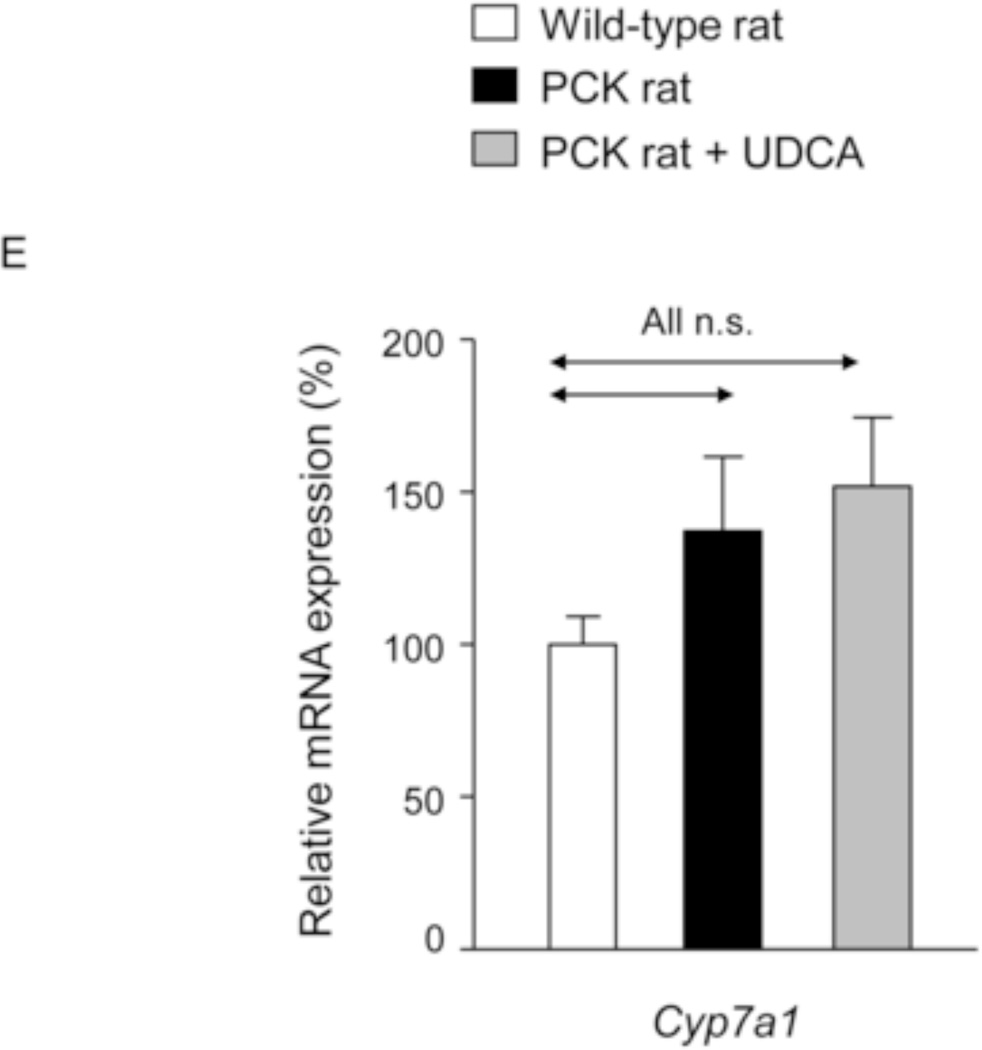
Figure 2.
PCK rats show increased intrahepatic bile acid concentration compared to wild-type rats, and UDCA decreases the intrahepatic accumulation of cytotoxic bile acids in PCK rats. (A) Total bile acid concentration ([BA]) in liver of wild-type and PCK rats (untreated and UDCA-treated). (B) Levels of both primary (CA and CDCA) and secondary (DCA and LCA) major species of BAs in liver of wild-type and PCK (untreated and UDCA-treated) rats. (C) Intrahepatic levels of the toxic dihydroxylated BAs (TDCA, TCDCA, GDCA and GCDCA) in wild-type and PCK (untreated and UDCA-treated) rats. (D) Proportion of unconjugated and, tauro- and glyco-conjugated BAs in wild-type and PCK (untreated and UDCA-treated) rats. (E) Expression level (mRNA) of Cyp7a1 in liver of wild-type and PCK (untreated and UDCA-treated) rats.
N=12 in wild-type (untreated) and n=10 in PCK (untreated and UDCA-treated) groups.
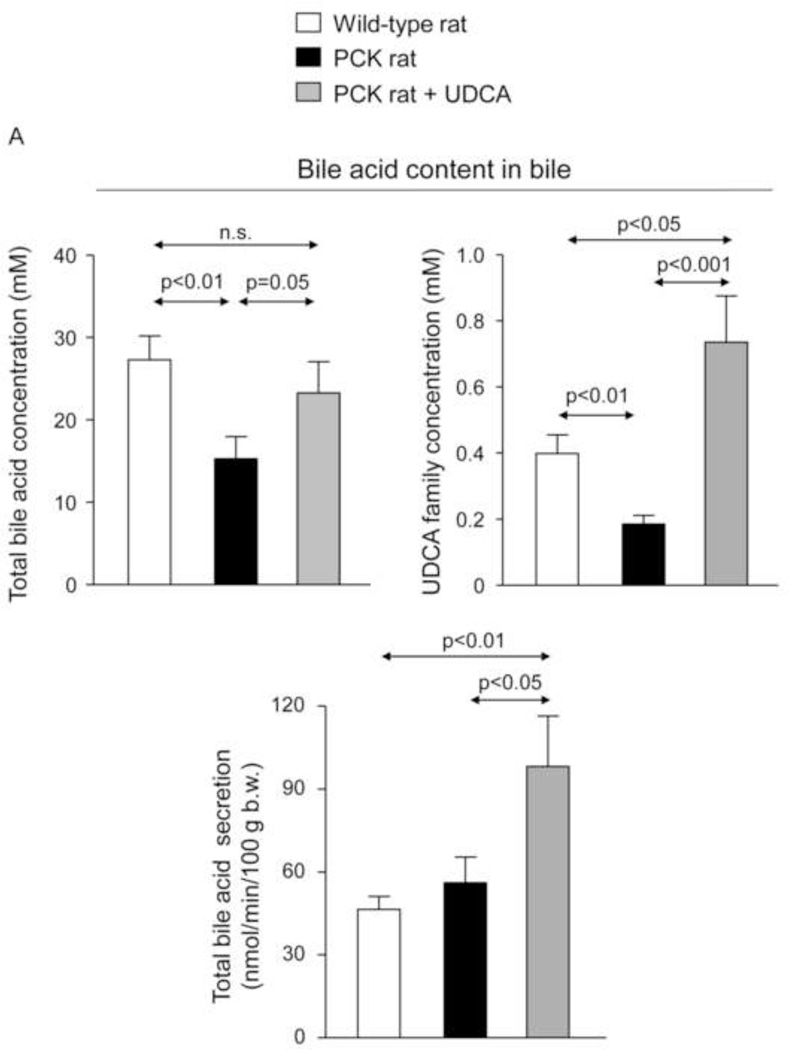
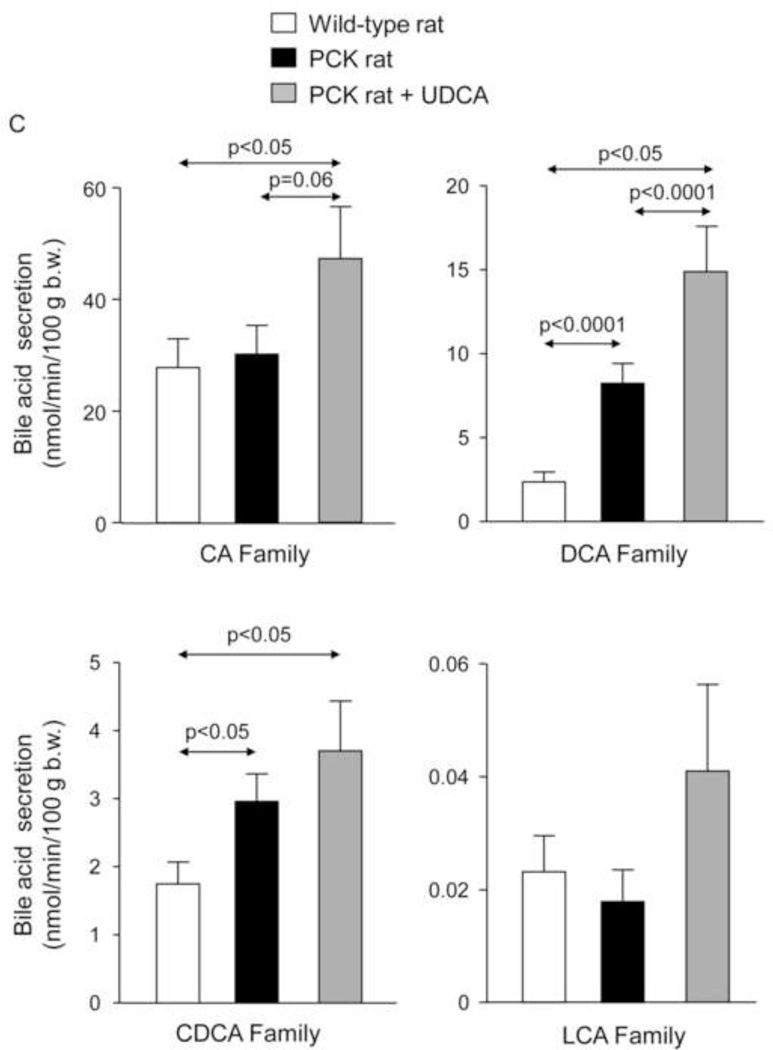
To investigate whether changes in BA synthesis could account for the increased intrahepatic accumulation of BAs, expression level of Cyp7a1, a key enzyme in BA synthesis, was determined. The abundance of Cyp7a1 mRNA was not different between wild-type and PCK rats, and was not affected by the treatment with UDCA (Fig. 2E), suggesting that changes in BA synthesis was probably not the cause of enhanced accumulation of these compounds in liver tissue. In addition, we measured the bile flow (Table 1) and collected bile samples from the common bile-duct before the animal´s sacrifice. The analysis revealed that total [BA] is lower in bile samples collected from PCK than wild-type rats (Figs. 3A,B); interestingly, UDCA treatment almost completely prevented this decreased [BA] (Figs. 3A,B and Suppl. Figs. 3A,B) and stimulated the BA secretion to bile in PCK rats (Figs. 3A,C).
Figure 3.
PCK rats show decreased [BA] in bile, and UDCA normalizes it and increases the BA secretion to bile. (A,B) [BA] in bile of wild-type and PCK (untreated and UDCA-treated) rats. (A,C) BA secretion to bile in wild-type and PCK (untreated and UDCA-treated) rats.
N=12 in wild-type (untreated) and n=10 in PCK (untreated and UDCA-treated) groups.
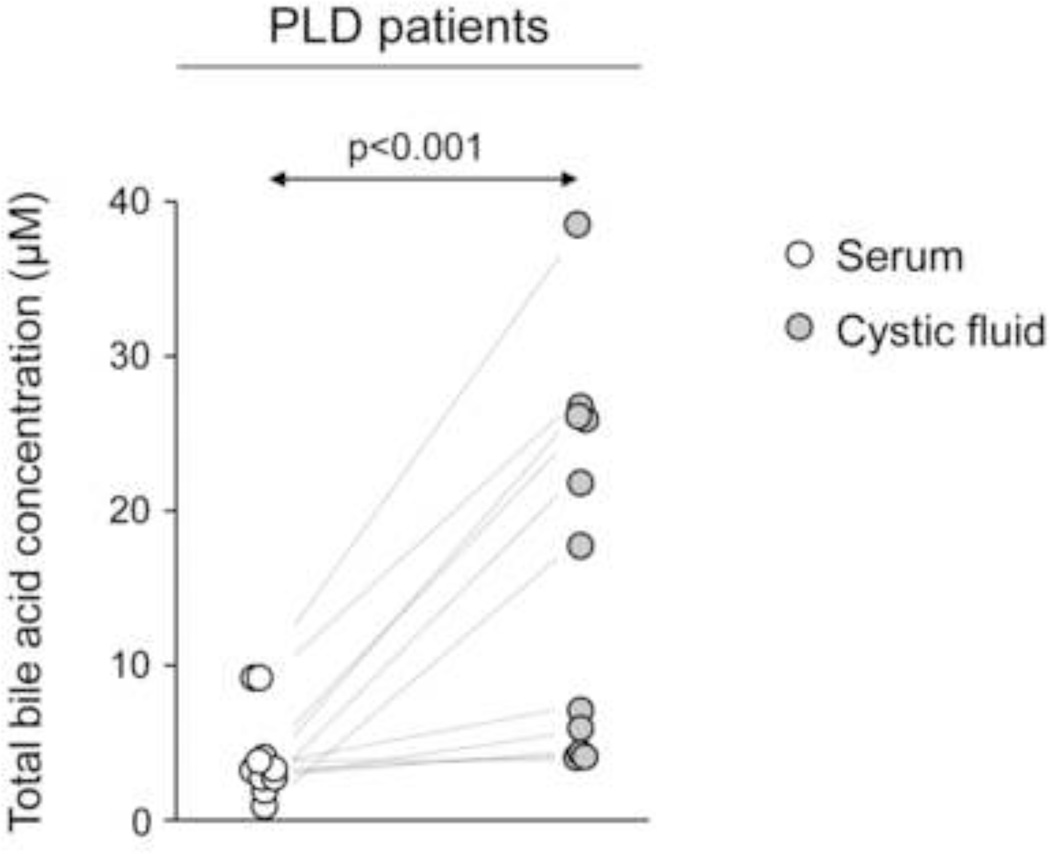
To evaluate where the BA accumulation takes place in polycystic livers, BA concentration was directly determined in the cystic fluid of PLD patients and compared with BA concentration in their paired samples of peripheral blood. Results show that BAs are significantly concentrated in cystic fluid compared with peripheral blood, although not as much as in bile. BA concentration in the cystic fluid is in “µM range” whereas in bile is in “mM range” (Fig. 4).
Figure 4.
The cystic fluid of PLD patients presents increased [BA] compared to their matched serum levels. Dots represent each patient (n=11).
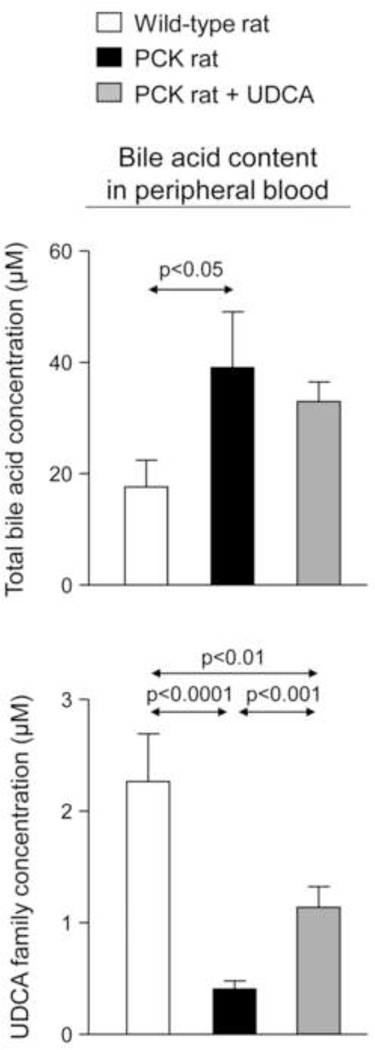
On the other hand, BA concentration is increased in peripheral blood of untreated PCK rats compared to wild-type rats, whereas UDCA family concentration is found decreased (Fig. 5 and Suppl. Figs. 4A–C). UDCA treatment upregulated UDCA family concentration in peripheral blood of PCK rats but level is still lower than that observed in wild-type rats (Fig. 5).
Figure 5.
PCK rats show increased total [BA] in peripheral blood compared to wild-type rats, as well as decreased UDCA family levels that are upregulated by UDCA treatment. N=12 in wild-type (untreated) and n=10 in PCK (untreated and UDCA-treated) rats.
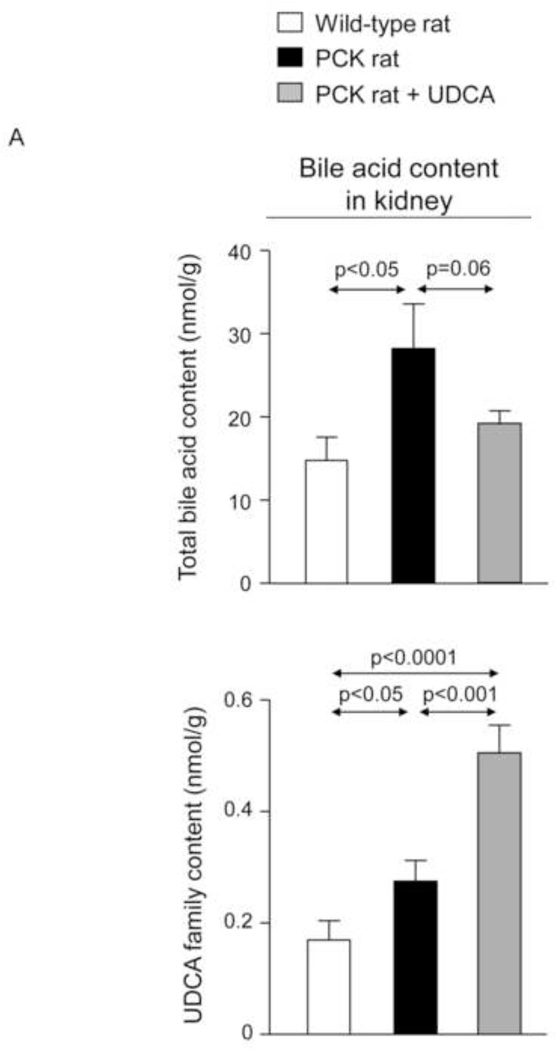
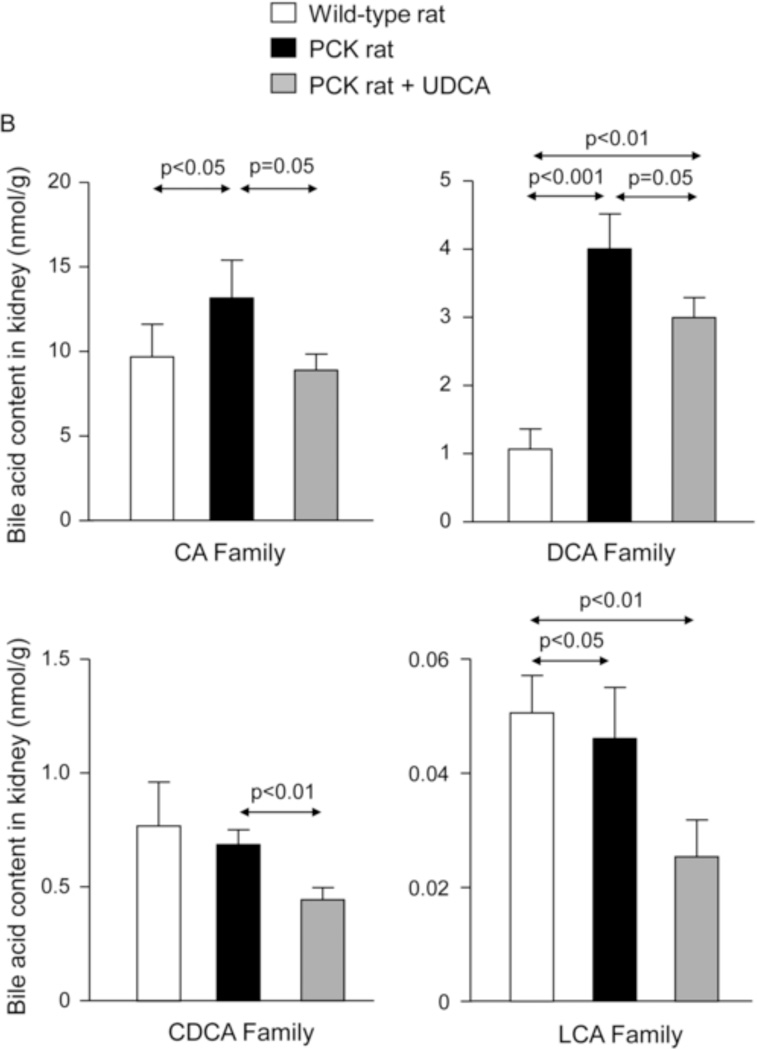
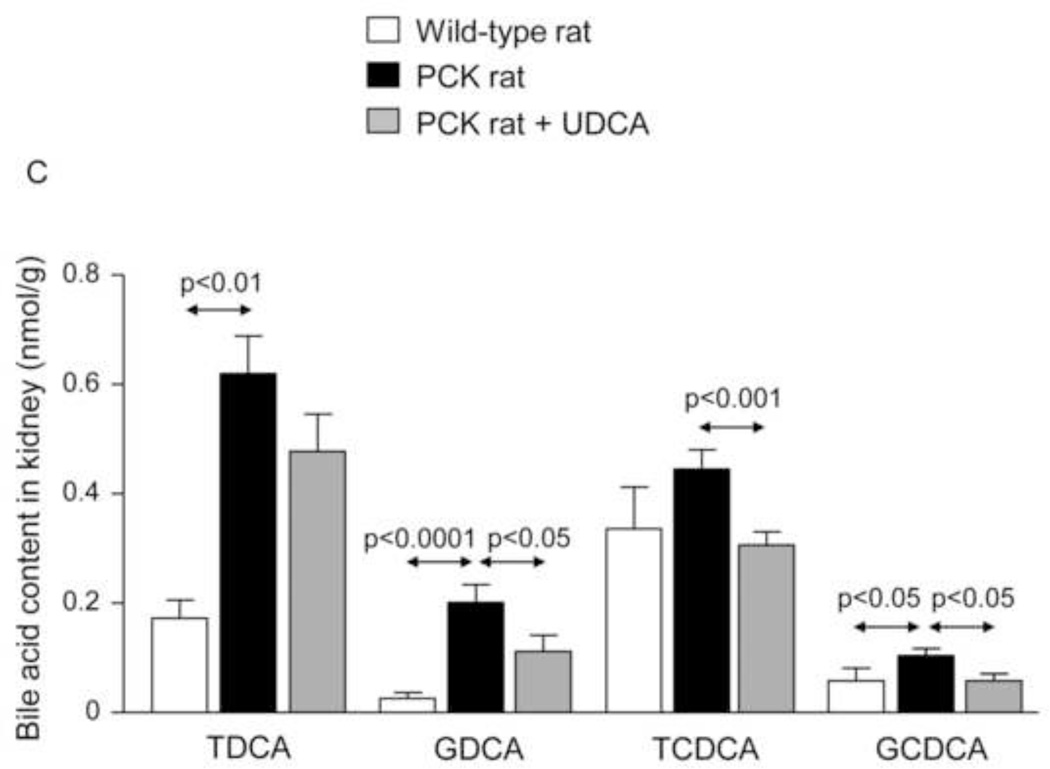
Moreover, BA concentration is increased in kidneys of non-treated PCK rats compared to wild-type rats (Fig. 6A–C and Suppl. Fig. 5). Treatment with UDCA diminished the concentration of different families of BAs in kidney, particularly the most cytotoxic (Fig. 6A–C and Suppl. Fig. 5).
Figure 6.
The [BA] is increased in kidneys of PCK rats compared to wild-type rats and is diminished by UDCA treatment (A, B, C). N=12 in wild-type (untreated) and n=10 in PCK (untreated and UDCA-treated) groups.
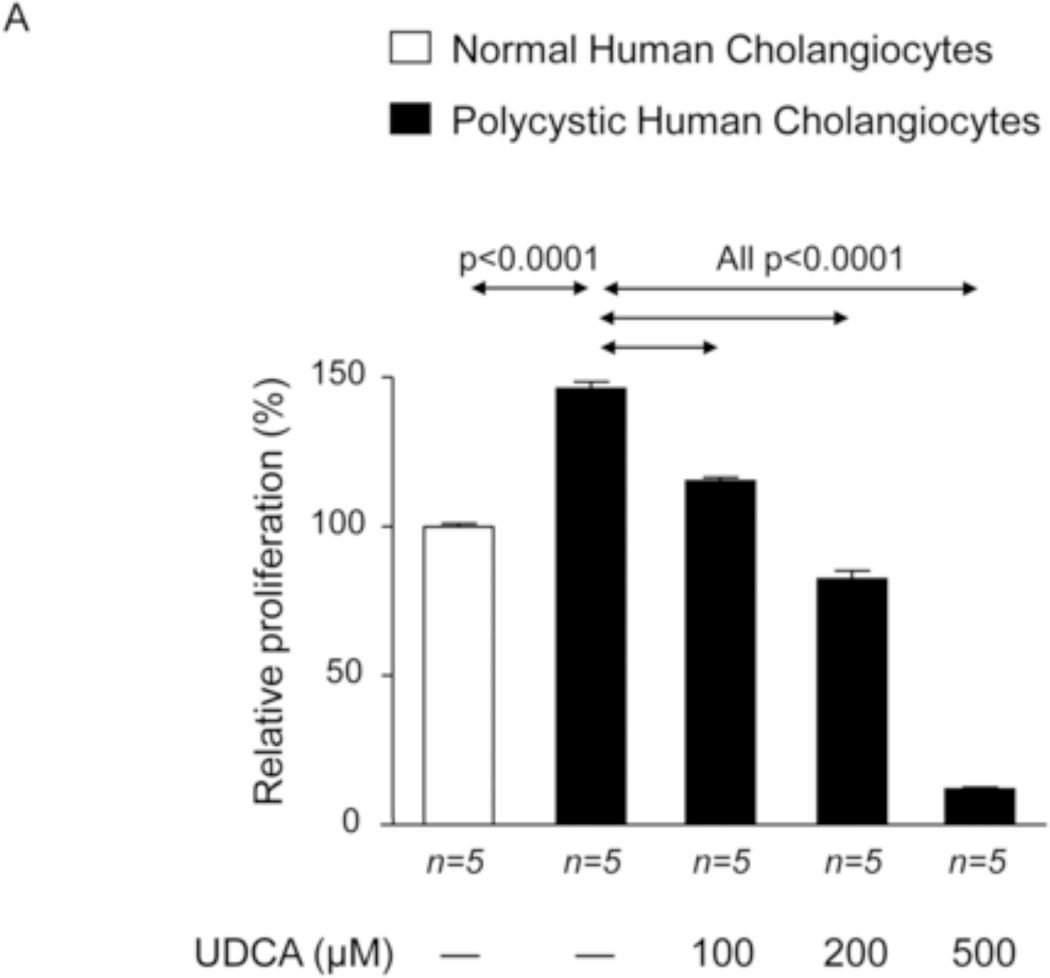
UDCA inhibits the proliferation of polycystic human cholangiocytes in culture
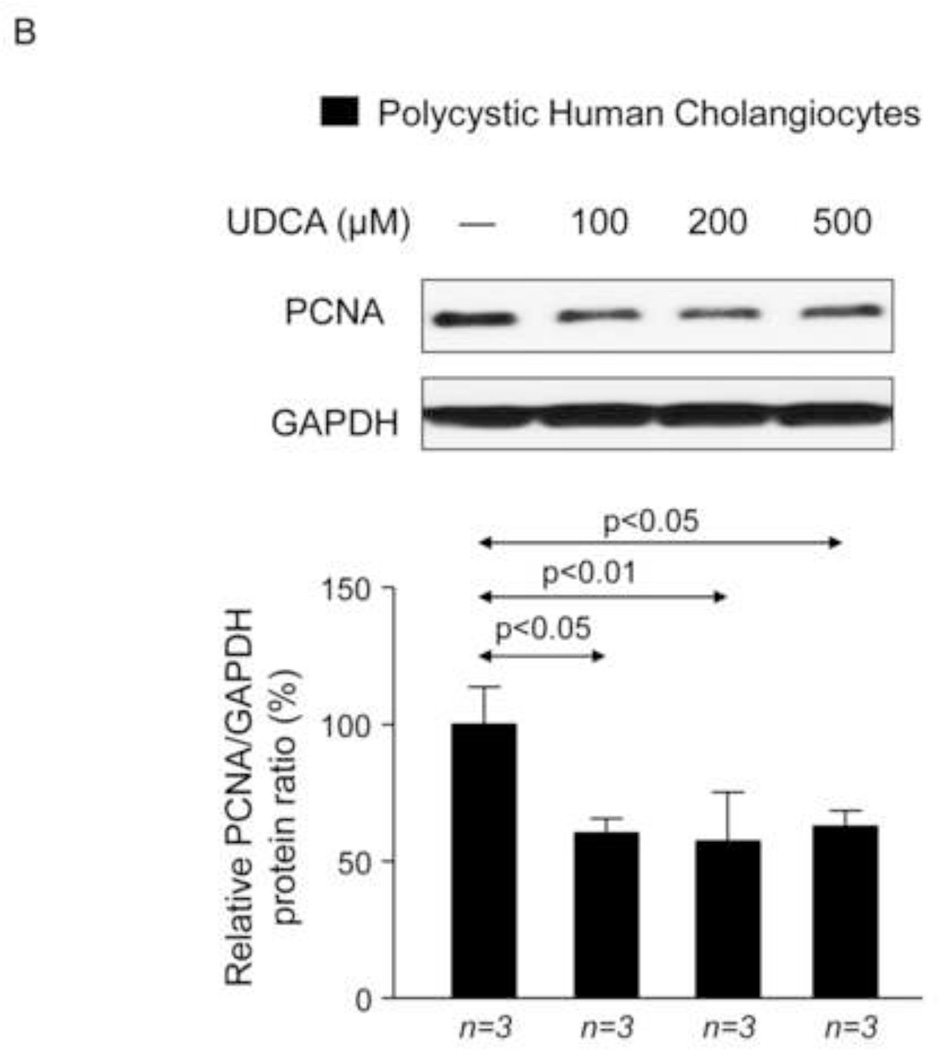
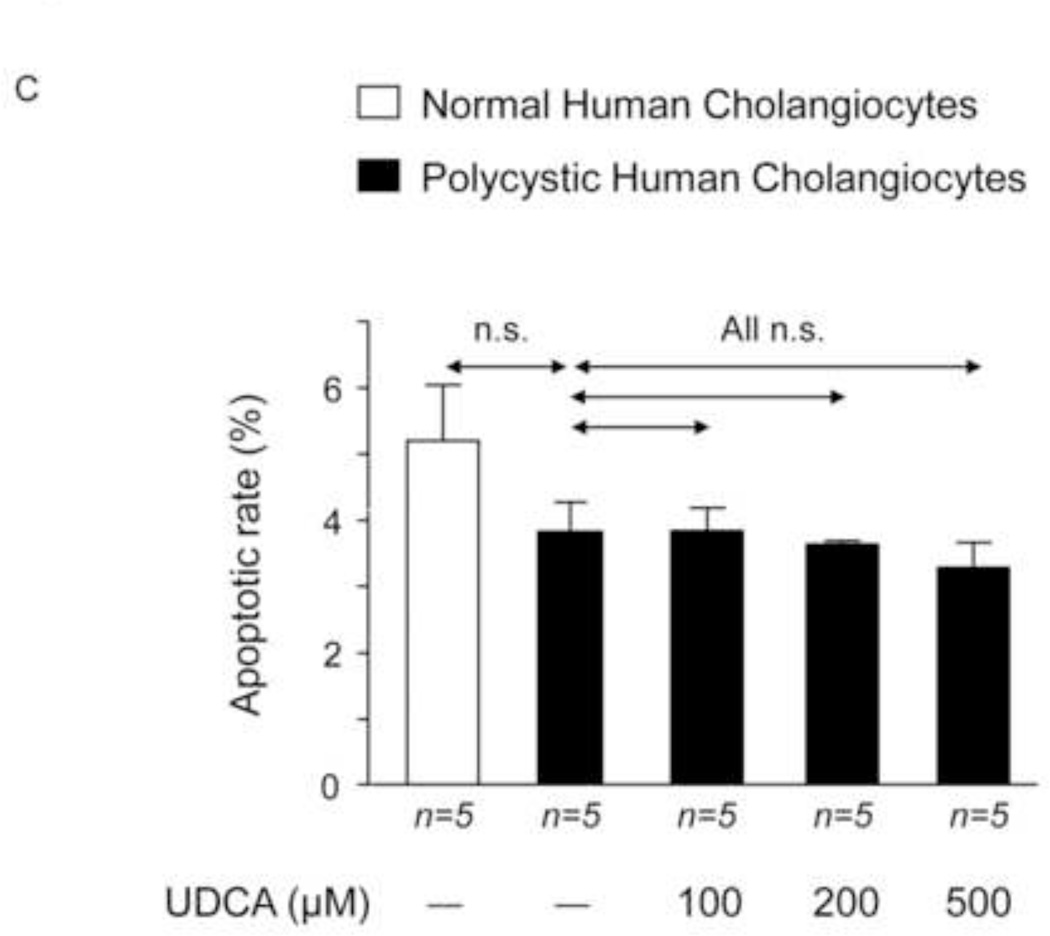
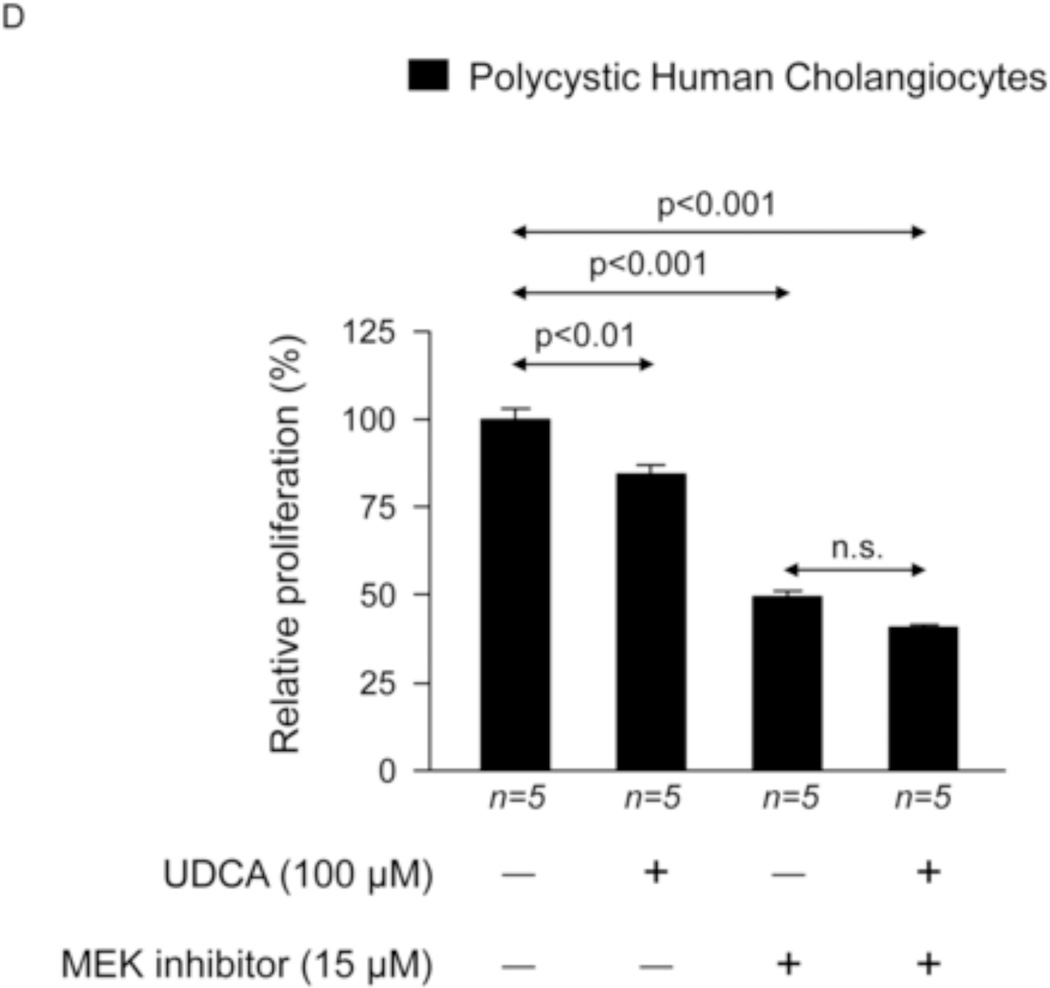
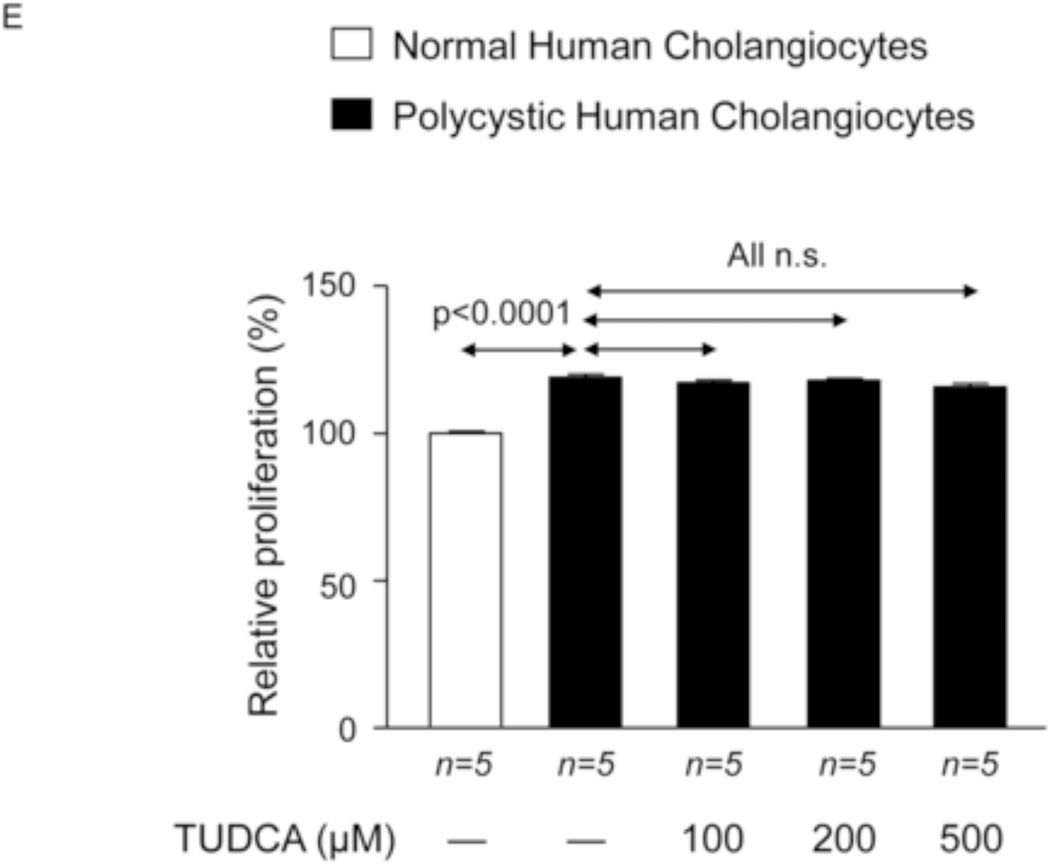
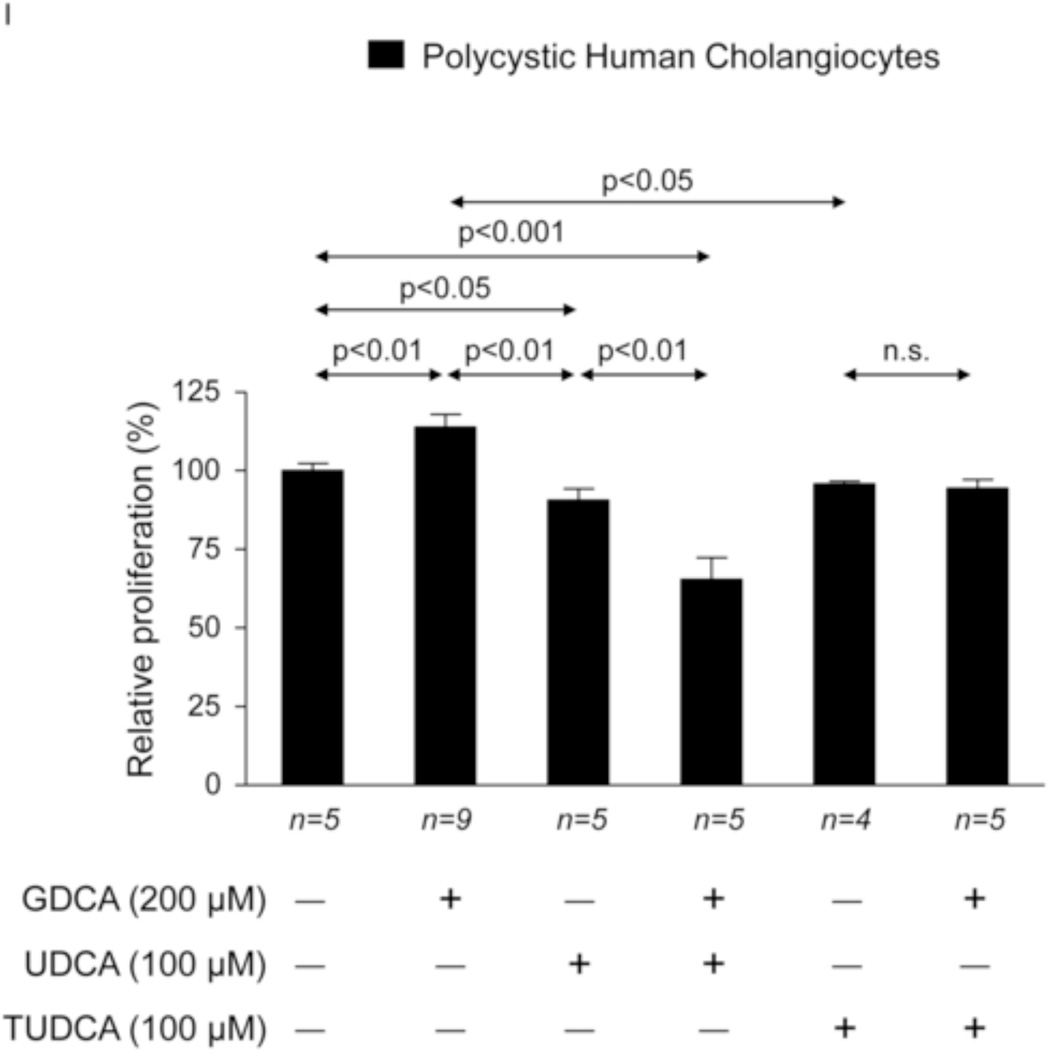
To investigate the potential direct effect of UDCA on biliary epithelia, several in vitro assays were carried out using polycystic human cholangiocytes. We first analyzed the basal proliferation rates of normal and polycystic human cholangiocyte cultures. Confirming previous results of our group in PCK rat cholangiocytes [11, 22], in the present study polycystic human cholangiocytes showed increased proliferation rates compared to normal human cholangiocytes in culture (Fig. 7A). In addition, the presence of UDCA in the culture medium inhibited such hyperproliferation in a dose-dependent manner (100, 200 or 500 µM) (Fig. 7A); this effect was associated with decreased protein levels of proliferating antigen PCNA (Fig. 7B) and with no changes in apoptosis (Fig. 7C). Moreover, the presence of a MEK inhibitor in the culture medium decreased the basal hyperproliferation of polycystic human cholangiocytes; interestingly, UDCA did not show an additive inhibitory effect with the MEK inhibitor indicating that its effect on proliferation is mediated, at least in part, by blocking the MEK pathway (Fig. 7D). Since UDCA is mainly conjugated with taurine in rat liver, the effect of TUDCA was evaluated on the basal hyperproliferation of polycystic human cholangiocytes in vitro. Our data indicate that TUDCA (100, 200 or 500 µM), in contrast to UDCA, does not alter the proliferation of polycystic human cholangiocytes (Fig. 7E)
Figure 7.
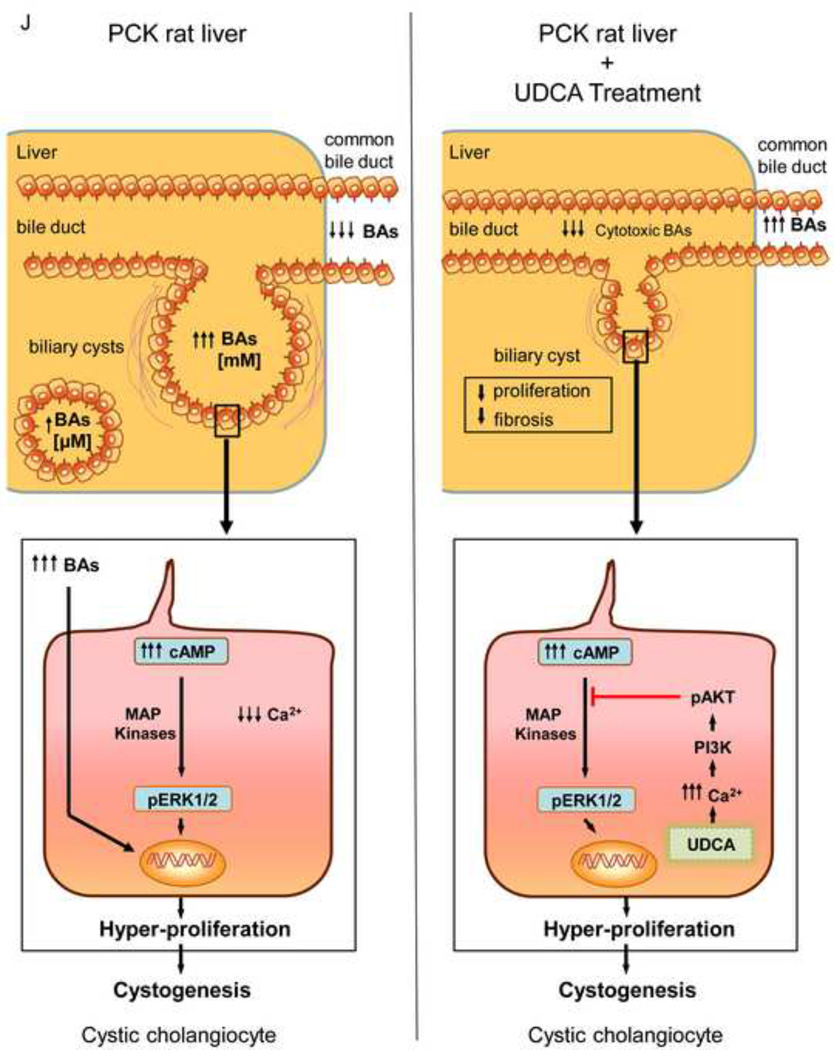
UDCA inhibits the proliferation of polycystic human cholangiocytes by raising the intracellular calcium levels and via a PI3K/AKT/MEK/ERK1/2-dependent mechanism. (A) Proliferation of normal and polycystic human cholangiocytes in the presence or absence of UDCA in the culture medium. (B) Protein expression of the pro-mitotic marker PCNA in polycystic human cholangiocytes in the presence or absence of UDCA in the culture medium (C) Basal apoptotic rates in normal and polycystic human cholangiocytes in the presence or absence of UDCA. (D) Role of MEK in basal and UDCA-inhibited proliferation of polycystic human cholangiocytes. (E) Role of TUDCA in the basal proliferation of polycystic human cholangiocytes. (F) Representative experiment and bar graph showing the intracellular calcium levels in normal and polycystic human cholangiocytes (n=number of cell groups analyzed). (G, H) Representative western blots and bar graphs showing AKT (G) and ERK1/2 (H) phosphorylation levels in the presence or absence of UDCA and/or PI3K-inhibitor. (I) Role of GDCA, UDCA and TUDCA in the proliferation of proliferation of polycystic human cholangiocytes. (J) Working model: hepatic cystogenesis in PLDs is characterized by cAMP/PKA/MEK/ERK1/2-dependent cholangiocyte hyperproliferation associated to decreased intracellular calcium level. The [BA] is increased in the liver of PCK rats and may promote the proliferation of polycystic cholangiocytes. UDCA inhibits the MEK/ERK1/2-dependent proliferation of polycystic cholangiocytes via Ca2+/PI3K/AKT mechanism, resulting in decreased hepatic cystogenesis and fibrosis. UDCA, through its choleretic features, may also flow the increased concentration of cytotoxic bile acids in the liver preventing their biliary pathogenic effects.
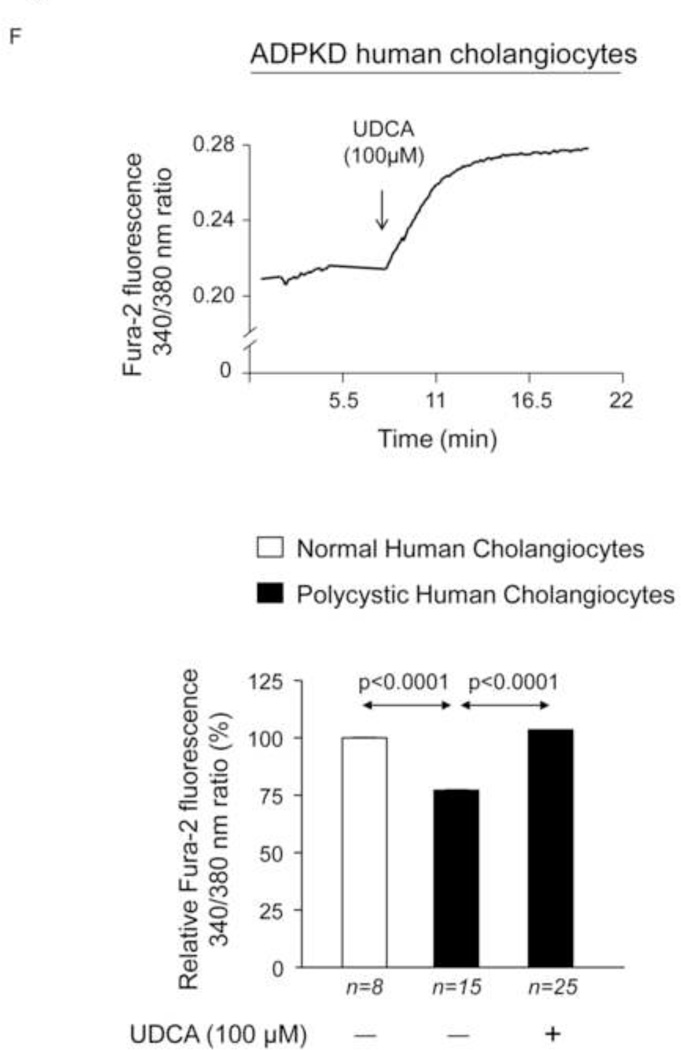
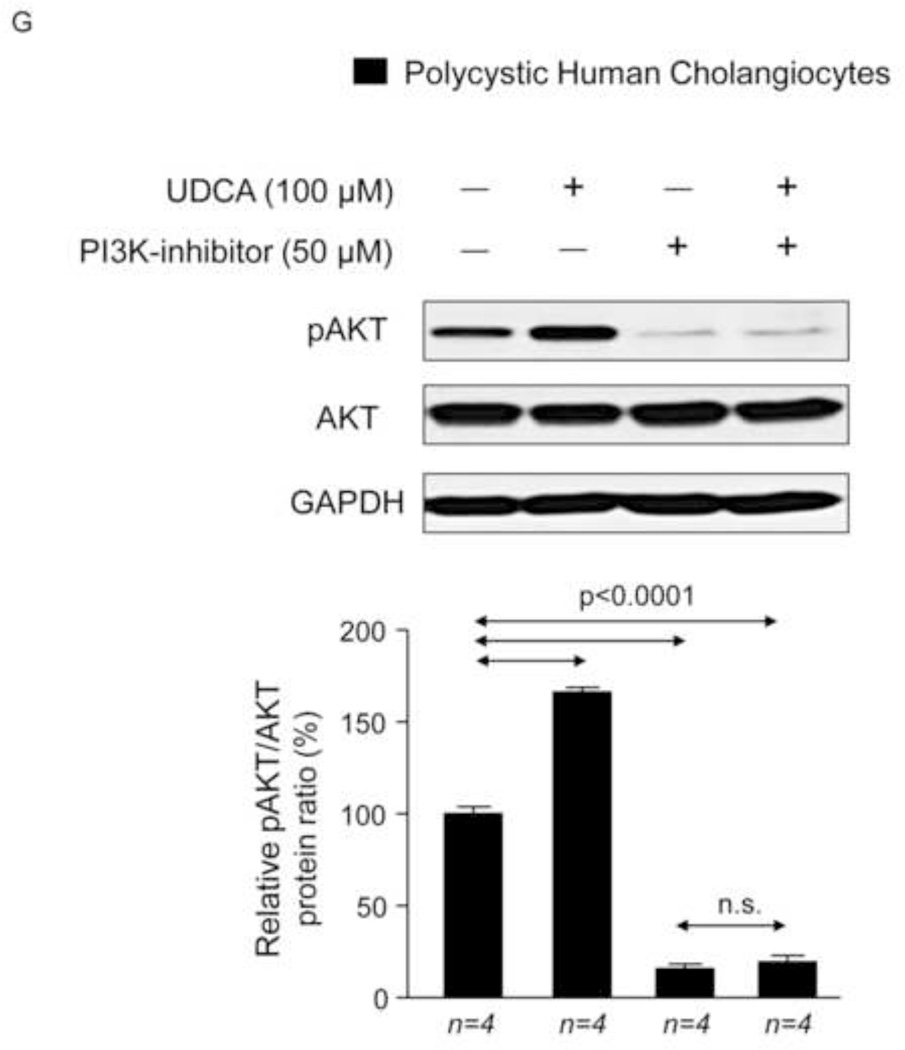
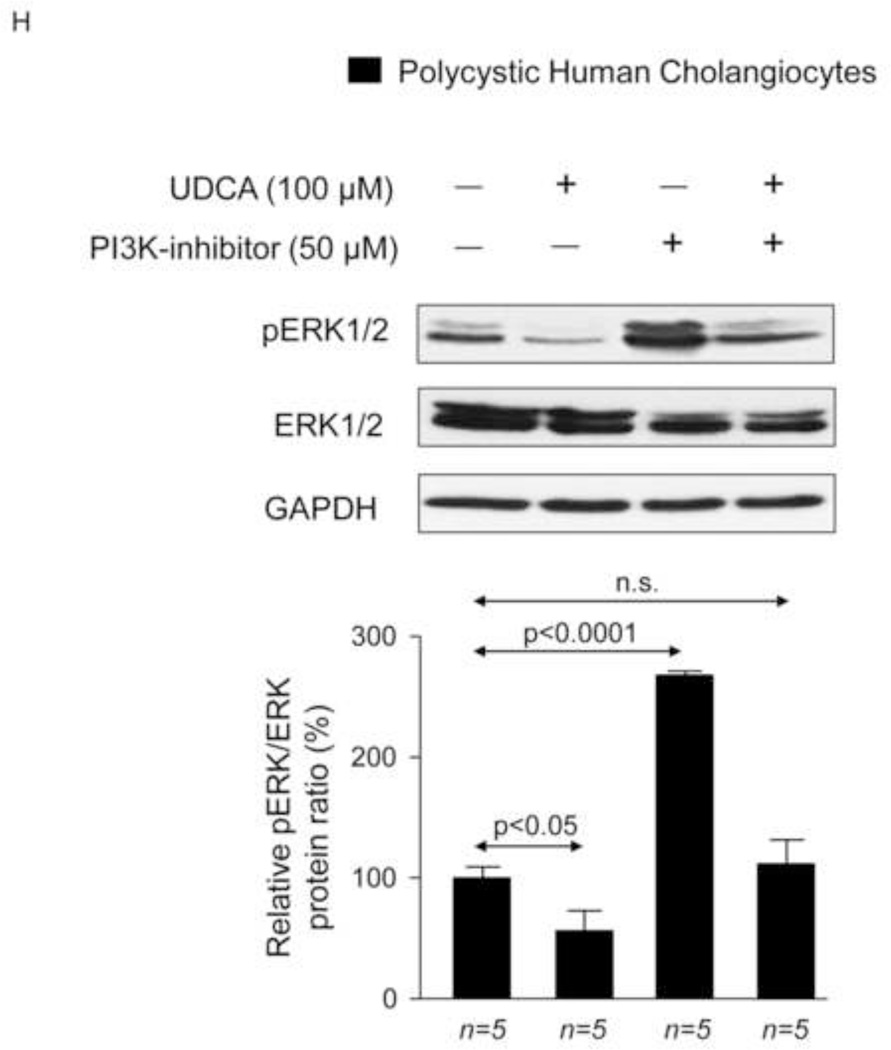
We previously reported that PCK rat cholangiocytes are characterized by decreased [Ca2+]i, which are responsible for both basal and cAMP/PKA/MEK/ERK1/2-dependent hyperproliferation through PI3K/AKT regulation [11]. Using microfluorimetric assays we have now observed that intracellular Ca2+ levels of polycystic human cholangiocytes are lower than those found in normal human cholangiocytes (Fig. 7F). Importantly, the presence of 100 µM UDCA in the culture medium normalized the diminished Ca2+ levels of cystic cholangiocytes (Fig. 7F) and stimulates AKT phosphorylation via a PI3K-dependent mechanism (Fig. 7G). In agreement with our previous observation on proliferation (Fig. 7C), UDCA decreases the ERK1/2 phosphorylation levels, and this effect is prevented by PI3K inhibition (Fig. 7H). Thus, UDCA-induced inhibition of cystic cholangiocyte proliferation is dependent on the rise of intracellular calcium levels that via a PI3K/AKT attenuates the MEK-dependent ERK1/2 phosphorylation and the subsequent proliferation.
Finally, the role of GDCA (a major secondary BA found concentrated in the liver of PCK rats) was evaluated on the proliferation of polycystic human cholangiocytes in vitro. The results indicate that GDCA stimulates the proliferation of these cells and this effect is inhibited by both UDCA and TUDCA (Fig. 7I).
Discussion
The key findings reported here suggest that UDCA may be a valuable therapeutic tool for the treatment of PLDs. Our data indicate that: i) treatment of PCK rats (8-week-old) with UDCA for 5 months decreases hepatic cystogenesis and fibrosis; ii) their motor behavior was also improved following UDCA treatment, suggesting a better physical fitness; iii) as compared to wild-type animals, PCK rats have increased [BA] in liver, similar hepatic Cyp7a1 mRNA levels, diminished [BA] in bile, and increased [BA] in peripheral blood and kidneys; iv) the cystic fluid of patients with PLD concentrates BAs compared to their matched serum levels; v) in PCK rats, UDCA decreases the intrahepatic accumulation of the most cytotoxic BAs, normalizes their diminished [BA] in bile, increases the BA secretion in bile and diminishes the increased [BA] in kidneys; vi) polycystic human cholangiocytes hyperproliferate as a consequence of diminished intracellular Ca2+ levels compared to normal human cholangiocyte cultures; vii) UDCA normalizes the intracellular calcium levels and inhibits the hyperproliferation of polycystic human cholangiocytes via a PI3K/AKT/MEK/ERK1/2-dependent mechanism without affecting apoptosis; viii) GDCA promotes the proliferation of polycystic human cholangiocytes, which is inhibited by both UDCA and TUDCA. These results are consistent with the notion that polycystic cholangiocytes hyperproliferate as a consequence of decreased intracellular calcium concentration and its restoration with UDCA may halt the hepatic cystogenesis of PLDs, representing a potential therapeutic tool.
We have previously reported that hepatic cystogenesis in PLDs may be a consequence of hyperproliferation [11], altered secretion [23], matrix-metalloprotease hyperactivity [18], centrosomal [24] and ciliary [22] abnormalities and global deregulation of microRNAs [25] in cholangiocytes, and that these cellular alterations may represent potential targets for therapy [2]. In this regard, we focused our attention on the characteristic hyperproliferation of cystic cholangiocytes and determined that this altered event is mediated by increased [cAMP]i in cholangiocytes [6]. Our previous published in vitro and in vivo data indicate that [cAMP]i is increased in PCK rat cholangiocytes compared to normal rat cholangiocytes, which stimulate their proliferation via a PKA/MEK/EKR1/2-dependent mechanism [6, 11]. Treatment of PCK rats with the somatostatin analog octreotide inhibited the hepatic cystogenesis through the downregulation of [cAMP]i in cholangiocytes and the concomitant hyperproliferation [6]. Those data led us and others to conduct clinical trials employing the somatostatin analogues octreotide [3–6] and lanreotide [7–9] with positive results in terms of reduced hepatic cystogenesis and improved life quality for patients. However, the inhibition of hepatic cystogenesis was modest (~5%). Thus, alternative approaches to guarantee a more pronounce and persistent inhibition of [cAMP]i in polycystic cholangiocytes are still needed.
We previously reported that PCK cholangiocytes are also characterized by decreased intracellular calcium level, and that its restoration with a calcium ionophore was able to block both basal and cAMP/PKA/MEK/ERK1/2-dependent hyperproliferation via PI3K/AKT activation [11]. As a consequence, the normalization of the [Ca2+]i in polycystic cholangiocytes may represent a valuable therapeutic approach for PLDs.
UDCA is an endogenous hydrophilic BA with hepato-protective features present in low concentration in humans [12, 13]. This BA stimulates the hepatobiliary secretion and protects both hepatocytes and cholangiocytes against the cytotoxicity of hydrophobic BAs through the biliary secretion of bicarbonate [12, 13]. At molecular level, UDCA mediates these effects in part by increasing the intracellular calcium levels [12, 13]. Interestingly, administration of UDCA to bile-duct ligated (BDL) rats (experimental model of cholestasis) resulted in inhibition of the characteristic cAMP-mediated cholangiocyte hyperproliferation via upregulation of the [Ca2+]i, which is abnormally diminished in BDL-cholangiocytes [14, 15]. Since hepatic cystogenesis in PLDs is also characterized by increased [cAMP]i and decreased [Ca2+]i in cystic cholangiocytes [11, 26], we hypothesized that UDCA could have therapeutic value for these genetic disorders. To test this theory, we conducted in vivo and in vitro studies using different experimental models of PLD. Chronic treatment of PCK rats with UDCA halted the development and progression of the liver disease compared to untreated PCK rats. Thus, inhibition of hepatic cystogenesis and fibrosis was observed in PCK rats treated with UDCA compared to controls. In addittion, we found that PCK rats have increased liver [BA]. Treatment of PCK rats with UDCA decreased the elevated levels of the most cytotoxic BAs. Since hepatic mRNA expression levels of CYP7A1 are not altered between PCK and wild-type rats, and no elevation of liver transaminases was found in PCK vs wild-type rats, we hypothesize that BAs are not hyperproduced and/or concentrated in hepatocytes of PCK rats. On the other hand, BAs may be concentrated in the bile of those cysts that are still connected to the biliary tree, and that UDCA, through its choleretic effect, may reduce this intrahepatic concentration of BAs. In this regard, cysts in the PCK rat usually appear in clusters connected to the biliary tree, which may partially separate and become isolated when the disease progresses. However, even in advanced stages of the liver disease, a certain proportion of cysts are still connected to the biliary tree [17]. In addition, our data show that BAs are significantly concentrated in cystic fluid of isolated cysts from PLD patients compared with their paired samples of peripheral blood. However, [BA] in the cystic fluid is in “µM range” whereas in bile this is “mM range”. Therefore, we believe that BA accumulation in the liver of PCK rats may mainly take place in two compartments: i) a proportion in isolated cysts with [BA] in “µM range” and ii) a certain proportion in cysts still connected to the biliary tree that contain [BA] in “mM range” and hence quantitatively play a major role in total amount of BAs retained in this organ. In addition, it is reasonable to hypothesize that those cysts that are still connected to the biliary tree may display slower drainage due to the architecture of the system and the presence of cellular and secretory debris that may partially block the exit of the cyst and hence the lavage of the content by the bile flow. The high [BA] within the cystic fluid may also be responsible of the hyperproliferation of cystic cholangiocytes in vivo, since we found that the major secondary bile acid GDCA stimulate the proliferation of polycystic human cholangiocytes in vitro. Based on these results, we further analyzed the potential direct beneficial effect of UDCA in cystic cholangiocytes and the molecular mechanisms involved. We found that polycystic human cholangiocytes, similarly to a previous report using cholangiocytes from PCK rats [11], have decreased intracellular calcium levels. UDCA normalizes the intracellular calcium levels and inhibits the proliferation of polycystic human cholangiocytes via a PI3K/AKT/MEK/ERK1/2-dependent mechanism without affecting apoptosis. In addition, the GDCA-stimulated proliferation of polycystic human cholangiocytes is inhibited by both UDCA and TUDCA. Therefore, UDCA might halt hepatic cystogenesis in vivo through both direct inhibition of cholangiocyte proliferation and by decreasing the intrahepatic concentration of certain pro-mitotic and cytotoxic BAs. It can be postulated that the benefits of UDCA on PCK rat physical status can be ascribed to the above described favourable effect on animal liver injury. Indeed, treatment with UDCA restored their mobility indicating better physical fitness.
In contrast, and as expected, chronic treatment of PCK rats with UDCA did not affect the renal cystogenesis that is also apparent in PCK rats [16]. However, UDCA diminished the increased [BA] in the kidneys of PCK rats, particularly the levels of the most cytotoxic, suggesting that UDCA may facilitate the exit of BAs in the kidney through the urine. Although kidneys of PCK rats treated with UDCA showed increased levels of UDCA compared to both non-treated PCK rats and wild-type rats, UDCA family levels are much lower than those found in the liver of treated animals. That might be the reason why UDCA shows no effect on renal cystogenesis, or perhaps UDCA does not regulate in the same way the proliferation of polycystic cholangiocytes and polycystic renal epithelial cells.
Altogether, these data strongly support the concept that UDCA may be a promising agent for the treatment of PLD patients. Oral administration of UDCA is well tolerated, safe (range between 13–25 mg/kg/day) and the only effective therapy approved by the U.S. Food and Drug Administration (FDA) for the treatment of chronic cholestatic disorders such as primary biliary cirrhosis (PBC) [27–29]. Among other disorders, UDCA is also recommended for cholesterol gallstone dissolution and hepatobiliary disorders associated with cystic fibrosis [30].
In summary (Fig. 7J), this mechanistic study provides pre-clinical evidence of the potential therapeutic role of UDCA for the treatment of PLD patients. Consequently, we have initiated an international multicenter phase II clinical trial (http://clinicaltrials.gov/show/NCT02021110) to evaluate this hypothesis. Future results will elucidate its potential use as monotherapy or in combination with somatostatin analogues or other new drugs for the treatment of PLDs.
Supplementary Material
Acknowledgments
Grant Support: Spanish Ministries of Economy and Competitiveness (J. M. Banales: FIS PI12/00380), and Science and Technology (J.J.G. Marin: SAF2010-15517 and SAF2013-40620-R), and “Instituto de Salud Carlos III” (J.M. Banales, L. Bujanda, JJ Marín, and J. Prieto: Ciberehd; J.M. Banales: Miguel Servet Program CON14/00129), Spain; Department of Industry of the Basque Government (J.M. Banales: SAIO12-PE12BN002), Spanish UTE for CIMA Project (J. M. Banales and J. Prieto), NIH of United States of America (N.F. LaRusso: DK24031) and Mayo Translational Polycystic Kidney Disease Center grant (T.V. Masyuk: P30-DK090728). JMB is funded by the “Asociación Española Contra el Cancer (AECC)”.
Abbreviations
- ADPKD
autosomal dominant polycystic kidney disease
- ADPLD
autosomal dominant polycystic liver disease
- AKT
v-akt murine thymoma viral oncogene homolog 1
- ALP
alkaline phosphatase
- α-Sma
alpha-smooth muscle actin
- ALT
alanine aminotransferase
- ARPKD
autosomal recessive polycystic kidney disease
- AST
aspartate aminotransferase
- BA
bile acid
- BDL
bile-duct ligation
- [Ca2+]I
intracellular calcium concentration
- CA
cholic acid
- cAMP
3'-5'-cyclic adenosine monophosphate
- CDCA
chenodeoxycholic acid
- CK19
cytokeratin 19
- Col1a1
collagen type 1 alpha 1
- Ctgf
connective tissue growth factor
- Cxcl1
C-X-C motif ligand 1 [interleukin 8 (IL8) homolog]
- Cyp7a1
cytochrome P450 7A1
- DCA
deoxycholic acid
- DMEM/F-12
Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12
- ERK1/2
extracellular signal-regulated kinases 1/2
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GCDCA
glycochenodeoxycholic acid
- GDCA
glycodeoxycholic acid
- IL6
interleukin 6
- LCA
lithocholic acid
- MAP kinases
mitogen-activated protein kinases
- MEK
mitogen-activated protein kinase kinase
- mRNA
messenger RNA
- pAKT
phosphorylated AKT
- PCK rat
polycystic kidney rat
- PLDs
polycystic liver diseases
- PCNA
proliferating cell nuclear antigen
- pERK
phosphorylated ERK
- PKA
protein kinase A
- PKDs
polycystic kidney diseases
- PKHD1
polycystic kidney and hepatic disease 1
- qPCR
quantitative polymerase chain reaction
- RT-PCR
reverse transcription polymerase chain reaction
- TCDCA
taurochenodeoxycholic acid
- TDCA
taurodeoxycholic acid
- Tgfβ1
transforming growth factor beta 1
- TUDCA
tauroursodeoxycholic acid
- UDCA
ursodeoxycholic acid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: authors disclose no conflicts.
Author Contributions: PMG, JJGM, MJP, ADU, OE, ES, MU, SS, AP, ARC, MRR, MJM, ASL, EH, RJA, TVM, MM, UB, NFL, JP, LB, JPHD, JMB: study concept and design, analysis and interpretation of data, drafting of the manuscript. PMG, JJGM, MJP, ADU, OE, ES, MU, SS, AP, ARC, MRR, MJM, ASL, EH, RJA, JMB: acquisition of data. PMG, JJGM, MJP, OE, ADU, ES, MU, MRR, MJM, ASL, JMB: statistical analysis. JJGM, NFL, TVM, JP, JPHD, LB, JMB: obtained funding
References
- 1.Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nature reviews Gastroenterology & hepatology. 2013;10(2):101–108. doi: 10.1038/nrgastro.2012.254. [DOI] [PubMed] [Google Scholar]
- 2.Perugorria MJ, Masyuk TV, Marin JJ, Marzioni M, Bujanda L, LaRusso NF, Banales JM. Polycystic liver diseases: advanced insights into the molecular mechanisms. Nature reviews Gastroenterology & hepatology. 2014;11(12):750–761. doi: 10.1038/nrgastro.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caroli A, Antiga L, Cafaro M, Fasolini G, Remuzzi A, Remuzzi G, Ruggenenti P. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5(5):783–789. doi: 10.2215/CJN.05380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan MC, Masyuk TV, Page L, Holmes DR, 3rd, Li X, Bergstralh EJ, et al. Somatostatin analog therapy for severe polycystic liver disease: results after 2 years. Nephrol Dial Transplant. 2012;27(9):3532–3539. doi: 10.1093/ndt/gfs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132(3):1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Chrispijn M, Nevens F, Gevers TJ, Vanslembrouck R, van Oijen MG, Coudyzer W, et al. The long-term outcome of patients with polycystic liver disease treated with lanreotide. Aliment Pharmacol Ther. 2012;35(2):266–274. doi: 10.1111/j.1365-2036.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 8.Temmerman F, Gevers T, Ho TA, Vanslembrouck R, Coudyzer W, van Pelt J, et al. Safety and efficacy of different lanreotide doses in the treatment of polycystic liver disease: pooled analysis of individual patient data. Aliment Pharmacol Ther. 2013;38(4):397–406. doi: 10.1111/apt.12384. [DOI] [PubMed] [Google Scholar]
- 9.van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137(5):1661–1668. doi: 10.1053/j.gastro.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 10.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, et al. Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology. 2013;58(1):409–421. doi: 10.1002/hep.26140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49(1):160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(6):318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 13.Poupon R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: an overview of their mechanisms of action. Clin Res Hepatol Gastroenterol. 2012;36(Suppl 1):S3–S12. doi: 10.1016/S2210-7401(12)70015-3. [DOI] [PubMed] [Google Scholar]
- 14.Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, et al. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 35(5):1041–1052. doi: 10.1053/jhep.2002.32712. 200. [DOI] [PubMed] [Google Scholar]
- 15.Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, et al. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. The American journal of pathology. 2006;168(2):398–409. doi: 10.2353/ajpath.2006.050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason SB, Liang Y, Sinders RM, Miller CA, Eggleston-Gulyas T, Crisler-Roberts R, et al. Disease stage characterization of hepatorenal fibrocystic pathology in the PCK rat model of ARPKD. Anat Rec (Hoboken) 2010;293(8):1279–1288. doi: 10.1002/ar.21166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masyuk TV, Huang BQ, Masyuk AI, Ritman EL, Torres VE, Wang X, et al. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. The American journal of pathology. 2004;165(5):1719–1730. doi: 10.1016/S0002-9440(10)63427-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urribarri AD, Munoz-Garrido P, Perugorria MJ, Erice O, Merino-Azpitarte M, Arbelaiz A, et al. Inhibition of metalloprotease hyperactivity in cystic cholangiocytes halts the development of polycystic liver diseases. Gut. 2014;63(10):1658–1667. doi: 10.1136/gutjnl-2013-305281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monte MJ, Martinez-Diez MC, El-Mir MY, Mendoza ME, Bravo P, Bachs O, Marin JJ. Changes in the pool of bile acids in hepatocyte nuclei during rat liver regeneration. J Hepatol. 2002;36(4):534–542. doi: 10.1016/s0168-8278(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 20.Nytofte NS, Serrano MA, Monte MJ, Gonzalez-Sanchez E, Tumer Z, Ladefoged K, et al. A homozygous nonsense mutation (c.214C->A) in the biliverdin reductase alpha gene (BLVRA) results in accumulation of biliverdin during episodes of cholestasis. J Med Genet. 2011;48(4):219–225. doi: 10.1136/jmg.2009.074567. [DOI] [PubMed] [Google Scholar]
- 21.Song C, Zhang XY, Manku M. Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyleicosapentaenoate treatment. J Neurosci. 2009;29(1):14–22. doi: 10.1523/JNEUROSCI.3569-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muff MA, Masyuk TV, Stroope AJ, Huang BQ, Splinter PL, Lee SO, Larusso NF. Development and characterization of a cholangiocyte cell line from the PCK rat, an animal model of Autosomal Recessive Polycystic Kidney Disease. Laboratory investigation; a journal of technical methods and pathology. 2006;86(9):940–950. doi: 10.1038/labinvest.3700448. [DOI] [PubMed] [Google Scholar]
- 23.Banales JM, Masyuk TV, Bogert PS, Huang BQ, Gradilone SA, Lee SO, et al. Hepatic cystogenesis is associated with abnormal expression and location of ion transporters and water channels in an animal model of autosomal recessive polycystic kidney disease. The American journal of pathology. 2008;173(6):1637–1646. doi: 10.2353/ajpath.2008.080125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masyuk TV, Lee SO, Radtke BN, Stroope AJ, Huang B, Banales JM, et al. Centrosomal abnormalities characterize human and rodent cystic cholangiocytes and are associated with Cdc25A overexpression. The American journal of pathology. 2014;184(1):110–121. doi: 10.1016/j.ajpath.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SO, Masyuk T, Splinter P, Banales JM, Masyuk A, Stroope A, Larusso N. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J Clin Invest. 2008;118(11):3714–3724. doi: 10.1172/JCI34922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spirli C, Locatelli L, Fiorotto R, Morell CM, Fabris L, Pozzan T, Strazzabosco M. Altered store operated calcium entry increases cyclic 3',5'-adenosine monophosphate production and extracellular signal-regulated kinases 1 and 2 phosphorylation in polycystin-2-defective cholangiocytes. Hepatology. 2012;55(3):856–868. doi: 10.1002/hep.24723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 27.De Marco G, Sordino D, Bruzzese E, Di Caro S, Mambretti D, Tramontano A, et al. Early treatment with ursodeoxycholic acid for cholestasis in children on parenteral nutrition because of primary intestinal failure. Aliment Pharmacol Ther. 2006;24(2):387–394. doi: 10.1111/j.1365-2036.2006.02972.x. [DOI] [PubMed] [Google Scholar]
- 28.Rost D, Rudolph G, Kloeters-Plachky P, Stiehl A. Effect of high-dose ursodeoxycholic acid on its biliary enrichment in primary sclerosing cholangitis. Hepatology. 2004;40(3):693–698. doi: 10.1002/hep.20370. [DOI] [PubMed] [Google Scholar]
- 29.Angulo P, Dickson ER, Therneau TM, Jorgensen RA, Smith C, DeSotel CK, et al. Comparison of three doses of ursodeoxycholic acid in the treatment of primary biliary cirrhosis: a randomized trial. J Hepatol. 1999;30(5):830–835. doi: 10.1016/s0168-8278(99)80136-6. [DOI] [PubMed] [Google Scholar]
- 30.Kotb MA. Molecular mechanisms of ursodeoxycholic Acid toxicity & side effects: ursodeoxycholic Acid freezes regeneration & induces hibernation mode. Int J Mol Sci. 2012;13(7):8882–8914. doi: 10.3390/ijms13078882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.