Abstract
A variety of CTX-M-type extended-spectrum β-lactamases (ESBLs), including hybrid ones, have been reported in China that are uncommon elsewhere. To better characterize the substrate profiles and enzymatic mechanisms of these enzymes, we performed comparative kinetic analyses of both parental and hybrid CTX-M enzymes, including CTX-M-15, -132, -123, -64, -14 and -55, that are known to confer variable levels of β-lactam resistance in the host strains. All tested enzymes were susceptible to serine β-lactamase inhibitors, with sulbactam exhibiting the weakest inhibitory effects. CTX-M-55, which differs from CTX-M-15 by one substitution, A77V, displayed enhanced catalytic activity (kcat/Km) against expanded-spectrum cephalosporins (ESCs). CTX-M-55 exhibits higher structure stability, most likely by forming hydrophobic interactions between A77V and various key residues in different helices, thereby stabilizing the core architecture of the helix cluster, and indirectly contributes to a more stable active site conformation, which in turn shows higher catalytic efficiency and is more tolerant to temperature change. Analyses of the hybrids and their parental prototypes showed that evolution from CTX-M-15 to CTX-M-132, CTX-M-123, and CTX-M-64, characterized by gradual enhancement of catalytic activity to ESCs, was attributed to introduction of different substitutions to amino acids distal to the active site of CTX-M-15. Similarly, the increased hydrolytic activities against cephalosporins and sensitivity to β-lactamase inhibitors, clavulanic acid and sulbactam, of CTX-M-64 were partly due to the amino acids that were different from CTX-M-14 and located at both the C and N termini of CTX-M-64. These data indicate that residues distal to the active site of CTX-Ms contributed to their enhanced catalytic activities to ESCs.
INTRODUCTION
The rapid dissemination of CTX-M-type extended-spectrum β-lactamases (ESBLs) in Enterobacteriaceae poses a huge threat to both human and animal health. To date, more than 150 different CTX-M-type ESBLs have been identified (http://www.lahey.org/studies/other.asp#table1). Based on the genetic relatedness, CTX-Ms can be divided into six clusters, including CTX-M-1, -2, -8, -9, and -25 and the KLUC groups (1). More than 95% identity is often observed within the same cluster, whereas less than 90% identity is detectable between clusters (2). Several hybrids of the CTX-M-1 and -9 groups, namely, CTX-M-64, CTX-M-123, CTX-M-137, and CTX-M-132, have also been identified recently (3–5). Formation of these hybrid CTX-M enzymes was suggested to be the result of recombination between blaCTX-M-15 and blaCTX-M-14, the two most dominant variants detectable worldwide (1, 6). However, the genetic environments of blaCTX-M-123 and blaCTX-M-64 were highly similar to that of blaCTX-M-55 identified in pHN1122-1, the second-most prevalent blaCTX-M variant in China (7). This suggests that recombination may take place between blaCTX-M-14 and blaCTX-M-55, producing various hybrid enzymes. Interestingly, all three published hybrids, CTX-M-64, CTX-M-123, and CTX-M-137, confer relatively high ceftazidime MICs compared to the majority of other CTX-M-type ESBLs (3–5). Conversely, the hydrolytic activities of CTX-M-55, CTX-M-123, and CTX-M-132 enzymes have not been described. Although the kinetic parameters of CTX-M-14, -15, and -64 have been reported, only a few substrates have been characterized (5, 8, 9). In addition, kinetic parameters of these enzymes for β-lactamase inhibitors and animal-use-only cephalosporins, namely, ceftiofur, are unknown. In this study, we performed comprehensive kinetic characterization of the major hybrids and their parental enzymes, including CTX-M-15, CTX-M-55, CTX-M-132, CTX-M-123, CTX-M-64, and CTX-M-14, with objectives of better understanding the mechanisms underlying the variable catalytic activities of these enzymes and providing insight into mechanisms of action of this class of β-lactamases.
MATERIALS AND METHODS
Antibiotics and chemicals.
Cefuroxime, ceftriaxone, ceftazidime, and cefotaxime were purchased from Sigma Chemical Co. (St. Louis, MO, USA), while cephalothin, ceftiofur, tazobactam, clavulanic acid, and sulbactam were purchased from Melonepharma Co. (Dalian, China). Ampicillin, kanamycin, and isopropyl-β-d-1-thiogalactopyranoside (IPTG) were purchased from IBI, Inc. (Boca Raton, FL, USA). Luria broth (LB) and nitrocefin were purchased from BD Co. (Franklin Lakes, NJ, USA). Mueller-Hinton broth (MHB) was purchased from Oxoid Co. (Hampshire, United Kingdom).
Bacterial strains and recombinant DNA.
Escherichia coli strains AHC4, carrying blaCTX-M-123 and blaCTX-M-55, and AHC46, carrying blaCTX-M-64, were isolated from chicken samples submitted to a veterinary diagnostic center in Anhui Province, China (4). E. coli strain LDH19 carrying blaCTX-M-132 was obtained from a urine sample from a female patient at a community hospital in Guangzhou, China. Clinical E. coli strains carrying blaCTX-M-14 and blaCTX-M-15 described in our previous study were also used in this study (10, 11). The blaCTX-M genes were amplified by PCR using primers listed in Table 1 and cloned into the pET28b vector (Novagen). Recombinant plasmids pET28-blaCTX-Ms were transformed into E. coli BL21(DE3) for CTX-M expression. Two versions of blaCTX-Ms were cloned, namely, those encoding the full-length precursor enzymes and enzymes without a signal peptide. E. coli carrying the full-length blaCTX-Ms was used to determine the MICs, whereas E. coli carrying the truncated versions of the blaH6-mCTX-M genes (without signal peptide regions; m represents mature form of CTX-M) was used for protein expression and purification.
TABLE 1.
Primers used to construct the CTX-M enzymes in this study
| Primer | Sequence |
|---|---|
| CTX-M-14-F | CCGGGATCCATGGTGACAAAGAGAGTGC |
| CTX-M-14-R | CGCGAATTCTTACAGCCCTTCGGCG |
| CTX-M-15/55/64/123/132-F | CCGGGATCCATGGTTAAAAAATCACTGC |
| CTX-M-15/55/64/123/132-R | RCGCGAATTCTTACAAACCGTCGGTGACG |
| mCTX-M-14-F | CCGGGATCCCAGACGAGTGCGGTGCAGC |
| mCTX-M-15/55/64/123/132-F | CCGGGATCCCAAACGGCGGACGTACAGC |
Antimicrobial susceptibility.
MICs of E. coli strains were determined for 7 β-lactams (ampicillin, cephalothin, cefuroxime, ceftiofur, ceftriaxone, cefotaxime, and ceftazidime) alone or in combination with a fixed, 4-μg/ml concentration of β-lactamase inhibitors (tazobactam, clavulanic acid, or sulbactam) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (12, 13). The strains were grown on a Mueller-Hinton agar (MHA) plate at 37°C overnight and transferred to MHB media supplemented with a serial concentration of selected β-lactams the next day, followed by incubation at 37°C for 16 to 20 h. The lowest concentrations of β-lactams that inhibited the growth of the E. coli cells were determined as the MICs. For E. coli BL21(DE3), the MICs toward different β-lactams were determined as aforementioned except in the presence of 1 mM IPTG in the MHB medium. Determination of MIC was repeated at least in triplicate for each antibiotic tested. E. coli strains (ATCC 25922 and ATCC 35218) were used as quality controls.
DNA and protein analyses.
The blaCTX-M genes and the phylogenetic tree were analyzed and constructed using SeqMan software, while the protein alignment was done using MegAlign software of Lasergene 7 (DNASTAR, Inc., WI, USA). The signal sequences of CTX-M enzymes were determined using the SignalP 4.1 server available at http://www.cbs.dtu.dk/services/SignalP/.
Overexpression and purification of CTX-M enzymes.
Freshly cultured E. coli BL21(DE3) carrying pET28-blaH6-mCTX-M, at a cell density of 0.6 at an optical density at 600 nm (OD600) was induced with 1 mM IPTG and continuously incubated at 30°C for 5 h. The cells were recovered, resuspended in lysis buffer (10 mM Tris-HCl [pH 7.6], 0.3 M sucrose, 1% NP-40, 0.5% Triton X-100, and 0.5% Tween 20), and broken with a French press (Thermo Scientific, Inc., MA, USA) by 3 passes at 20,000 lb/in2 with sample cooling in an ice-water bath for 1 min after each pass. The soluble fractions were loaded onto a Ni-nitrilotriacetic acid (NTA) column, washed, and eluted with a binding buffer of 20 mM Tris-HCl, pH 7.9, and 500 mM NaCl containing different concentrations of imidazole. Briefly, the Ni-NTA column was washed twice with binding buffers containing 10 mM and 20 mM imidazole. The His6-tagged proteins were then eluted with binding buffer containing 100 mM imidazole, followed by concentration using an Amicon ultra-15 (nominal molecular weight limit, 10) centrifugal filter device, and the buffer was exchanged with 50 mM phosphate buffer, pH 7.4, during the process. The His6 tag was removed by incubating the purified enzyme with thrombin at a ratio of 100 μg protein per unit of thrombin at room temperature for 1 h. The thrombin-treated proteins were further subjected to gel filtration chromatography on an Sephacryl S-200 column. Fractions containing the mCTX-M were pooled and concentrated using the Amicon ultra-15 centrifugal filter device. The purity of mCTX-M proteins was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis and determined as at least 99% pure for all of the mCTX-M enzymes purified. The concentrations of enzymes were determined using Nanodrop Lite (Thermo Scientific, Inc., MA, USA). The yields for the six mCTX-M enzymes in this study ranged from 14 to 15.2 mg/500 ml.
Western blot.
The expression levels of CTX-M enzymes were verified by Western blotting. In brief, E. coli cultures carrying pET28-blaH6-mCTX-M were induced with 1 mM IPTG, as described previously. The cells were recovered and resuspended in B-per buffer (Thermo Scientific, Inc., MA, USA). The obtained total lysate were loaded onto an SDS-PAGE, followed by transfer to a nitrocellulose membrane. The membrane was probed with anti-H6 antibody to check the expression levels of E. coli cultures carrying different CTX-M enzymes. Anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody was probed to ensure the equal loading.
Determination of kinetic constants and IC50.
The kinetic parameters of mCTX-Ms were determined by mixing the enzyme with different concentrations of β-lactams at 25°C in the assay buffer (50 mM phosphate buffer, pH 7.0) without preincubation. The initial velocities of substrate hydrolysis for ampicillin (Δε235 = −820 M−1 cm−1), nitrocefin (Δε482 = 15,000 M−1 cm−1), cephalothin (Δε260 = −6,500 M−1 cm−1), cefuroxime (Δε260 = −7,600 M−1 cm−1), ceftriaxone (Δε260 = −10,351 M−1 cm−1), cefotaxime (Δε260 = −7,500 M−1 cm−1), ceftiofur (Δε260 = −5,614 M−1 cm−1), and ceftazidime (Δε260 = −9,000 M−1 cm−1) were measured by monitoring the changes of absorbance in a 1-cm quartz cuvette by a spectrometer (PerkinElmer Lambda Bio20) for 5 min (14). The molar extinction coefficients for ceftriaxone and ceftiofur were determined in our lab, while the others were obtained from a previous study (data not shown) (14). The initial velocities obtained from the substrate range of 0.2 to 2× Km were measured in at least triplicate and fitted to the nonlinear regression of the Michaelis-Menten equation using least-squares (ordinary) fit by GraphPad Prism5 (San Diego, CA, USA) to determine the Km, kcat, and standard errors. Bovine serum albumin (BSA) (100 μg/ml) was added to the diluted enzymes and the reaction mixture to prevent enzyme degradation. For ceftazidime, which was a poor substrate, the Ki values of mCTX-Ms enzymes were determined by a competitive inhibition assay using 100 μM nitrocefin as a reporter substrate (5, 15). The effect of temperature on the enzymatic activity was studied by determination of the kinetic constants of CTX-M-15 and CTX-M-55 toward cefotaxime using the same assay buffer and condition as aforementioned except at temperatures of 25°C, 40°C, and 60°C.
The 50% inhibitory concentration (IC50) was determined as the concentration of β-lactamase inhibitors (clavulanic acid, tazobactam, and sulbactam) required to reduce the hydrolysis rate of nitrocefin by 50% when the enzyme was preincubated with various concentrations of the inhibitor for 5 min at 25°C prior to addition of substrate.
Circular dichroism.
The thermal stability assay was performed by using 0.47 μM CTX-M-15 and CTX-M-55 in milli-Q water in a Jasco-J810 spectropolarimeter (Easton, MD, USA) at 25°C, 40°C, and 60°C. Quartz cells with a 10-mm path length were used for all experiments. Circular dichroism (CD) spectra were obtained and recorded every 0.1 nm between λ190 and λ250 with a scan rate of 50 nm/min and a 2-s response time. Data were measured in triplicate, and the CD signals were expressed as molar ellipticity.
Structural modeling.
Structures of CTX-M variants were generated using their amino acid sequences (GenBank accession numbers AEX93385.1, ACU87987.1, AGX00969.1, and JX313020 for CTX-M-55, -64, -123, and -132, respectively) and the comparative protein modeling Swiss-Model server available at http://swissmodel.expasy.org, employing 4HBT (for CTX-M-55, -123, and -132) and 1YLT (for CTX-M-64), of which both are CTX-M β-lactamases, as the templates. The Global Model Quality Estimation (GMQE) scores, the values used to estimate the accuracy of a model built with the template, were between 0.93 and 0.97 for the models built in this study. The protein structures and built models were analyzed by PyMOL.
RESULTS AND DISCUSSION
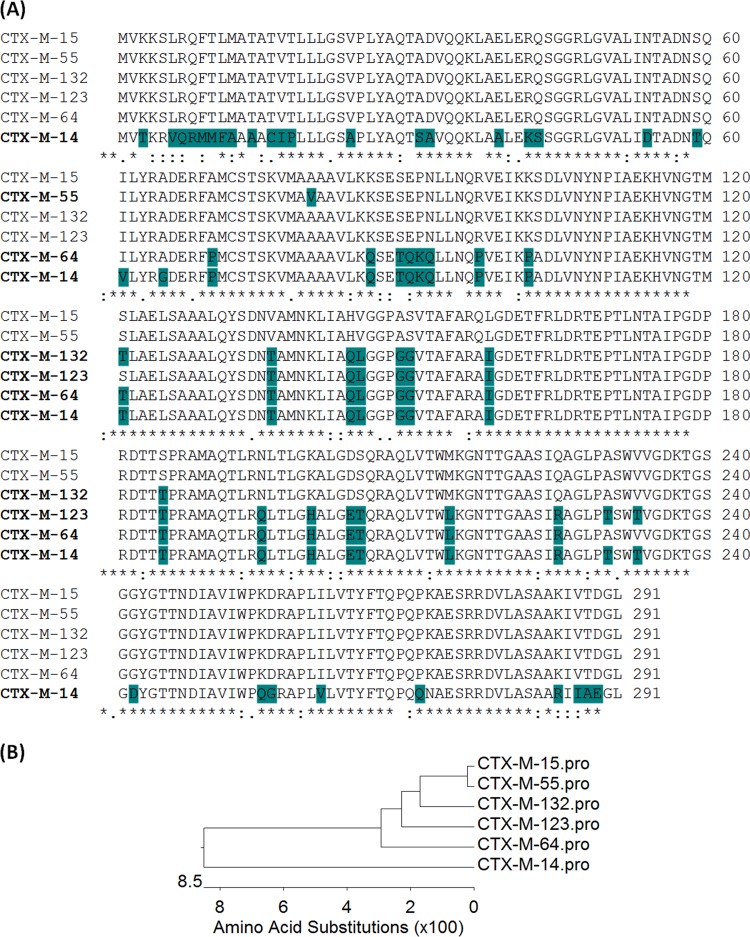
In this study, we aimed to characterize the catalytic activities and functional mechanisms of the two most prevalent CTX-M types of β-lactamases, CTX-M-14 and CTX-M-15, and the related hybrid enzymes reported recently, including CTX-M-132, CTX-M-123, and CTX-M-64. CTX-M-55 was also included in the characterization, since this variant exhibits a close relationship with CTX-M-15 and is generally regarded as the parental enzyme from which the hybrids evolved. Phylogenetic and sequence alignment analysis showed that CTX-M-14 and CTX-M-15 were the most divergent enzymes that comprised two distinct clusters, whereas CTX-M-55 differed from CTX-M-15 by only one residue, V77A (Fig. 1). Among the hybrids, CTX-M-132 was genetically closest to CTX-M-15, followed by CTX-M-123 and CTX-M-64, which were more closely related to CTX-M-14 (Fig. 1).
FIG 1.
Sequence alignment of CTX-M-14, CTX-M-15, CTX-M-55, and various related hybrids. (A) The alignment of different CTX-M enzymes. The amino acids are numbered by the standard numbering scheme for the class A β-lactamases according to Ambler et al. (23). (B) Phylogenetic tree of the 6 CTX-M variants used in this study.
To directly compare the phenotypes of these 6 different CTX-Ms and rule out other factors that may contribute to resistance for β-lactams, MICs of E. coli strains producing the full-length CTX-Ms, including the clinical strains and the pET28b clones toward different β-lactams, were determined (Table 2). The levels of expression in the total cell lysate for NH-mCTX-Ms (where NH is the N-terminal His tag) in E. coli were determined by probing the total lysate with anti-His6-tag antibody. As shown in Fig. 2, these 6 CTX-Ms showed similar expression levels. In terms of in vivo activities against different β-lactams tested, the order of the levels of MICs to E. coli carrying these blaCTX-Ms were as follows: ampicillin, cefuroxime > cephalothin, ceftiofur, ceftriaxone, and cefotaxime > ceftazidime. These CTX-Ms were susceptible to all three serine-based β-lactamase inhibitors, clavulanic acid, tazobactam, and sulbactam; sulbactam was the weakest inhibitor (Table 2). Regarding the MICs of different β-lactams for E. coli producing different CTX-Ms, CTX-M-14 showed the lowest MICs to most of the β-lactams tested, except ampicillin, compared to other CTX-Ms (Table 2). Among the other 5 CTX-Ms, CTX-M-64 exhibited the highest MICs for cefotaxime, whereas CTX-M-64, CTX-M-132, CTX-M-123, and CTX-M-55 conferred higher MICs for ceftazidime and cefotaxime than those mediated by CTX-M-15. These data suggested that CTX-M-64 showed relatively higher MICs for different β-lactams than other enzymes, such as CTX-M-132, CTX-M-123, and CTX-M-55, which in turn conferred higher MICs than CTX-M-15 (Table 2). All three commercially available serine β-lactamase inhibitors, clavulanic acid, tazobactam, and sulbactam, were very effective when used in a combinatorial manner with cefotaxime, with the activity of cefotaxime exhibiting the highest degree of reduction (up to 70,000-fold) compared to cefotaxime alone.
TABLE 2.
MICs of E. coli isolates carrying various blaCTX-Ms toward different β-lactams
| E. coli strain | blaCTX-M | MIC (μg/ml)a for: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | CEP | CFX | CTO | CRO | CAZ | CTX | CTX + TZBb | CTX + CLAb | CTX + SULb | ||
| Parental strain | M-15 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | 32 | ≥2,048 | 0.25 | 0.25 | 0.125 |
| M-55 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | 64 | ≥2,048 | 0.5 | 0.5 | 2 | |
| M-132 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | 64 | ≥2,048 | 0.06 | 0.125 | 2 | |
| M-123 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | 32 | ≥2,048 | 0.125 | 0.125 | 4 | |
| M-64 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | ≥2,048 | 64 | ≥2,048 | 0.125 | 0.125 | 8 | |
| M-14 | ≥2,048 | 1,024 | 1,024 | 512 | 1,024 | 4 | 256 | 0.06 | 0.06 | 0.125 | |
| BL21(DE3) carrying pET28b-blaCTX-M | M-15 | ≥2,048 | 256 | ≥2,048 | 512 | 256 | 2 | 128 | <0.015 | <0.015 | 0.06 |
| M-55 | ≥2,048 | 256 | ≥2,048 | 512 | 512 | 16 | 256 | <0.015 | <0.015 | 0.06 | |
| M-132 | ≥2,048 | 512 | ≥2,048 | 512 | 512 | 32 | 512 | <0.015 | <0.015 | 0.5 | |
| M-123 | ≥2,048 | 512 | ≥2,048 | 512 | 512 | 32 | 512 | <0.015 | <0.015 | 0.125 | |
| M-64 | ≥2,048 | 256 | ≥2,048 | 512 | 512 | 32 | 1024 | <0.015 | <0.015 | 0.125 | |
| M-14 | ≥2,048 | 256 | 1,024 | 128 | 256 | 0.5 | 128 | <0.015 | <0.015 | 0.5 | |
| M-14(D240G) | ≥2,048 | 256 | 1,024 | 128 | 256 | 0.5 | 256 | <0.015 | <0.015 | 0.5 | |
| E. coli BL21(DE3) | 16 | 16 | 4 | 0.03 | 0.03 | 0.03 | 0.06 | ||||
AMP, ampicillin; CEP, cephalothin; CFX, cefuroxime; CTO, ceftiofur; CRO, ceftriaxone; CAZ, ceftazidime; CTX, cefotaxime; CLA, clavulanic acid; TZB, tazobactam; SUL, sulbactam.
TZB, CLA, and SUL were each at a fixed concentration of 4 μg/ml.
FIG 2.
Protein expression levels of CTX-Ms by Western blotting. (A) The total lysate of E. coli carrying blaH6-mCTX-Ms was probed with anti-H6 antibody as described in Materials and Methods. (B) The expression levels were normalized by quantifying the bands using AlphaEaseFC.
Enzyme kinetic characterization of these CTX-Ms provided a clearer and better understanding of the differences between the catalytic activities of these enzymes than MICs, since one may doubt that the latter could be partially attributed to the high concentration of these enzymes in the periplasmic space. Similar to the MIC results, CTX-M-14 exhibited the lowest catalytic activity toward the β-lactams tested, except cephalothin. CTX-M-15 displayed slightly lower catalytic activities to most of the β-lactams tested, except ampicillin, compared to CTX-M-55 and the other three hybrids (Table 3). Consistent with the MIC data, CTX-M-55 exhibited similar catalytic activity as CTX-M-132 and CTX-M-123 for most of the β-lactams tested, except ampicillin, whereas CTX-M-64 exhibited the highest catalytic activity toward nitrocefin, cefuroxime, ceftiofur, ceftriaxone, and cefotaxime (Table 3). The IC50 and Ki of clavulanic acid, tazobactam, and sulbactam showed that all these three inhibitors displayed nanomolar levels of inhibition of these CTX-Ms, with sulbactam exhibiting higher IC50 and Ki than clavulanic acid, which was in turn slightly higher than tazobactam (Table 4). Such findings were consistent with the MIC data and previous studies (8, 13).
TABLE 3.
Kinetic constants of six purified CTX-M enzymes
| CTX-M enzyme | Constant | Value for: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| AMP | NCFa | CEP | CFX | CTO | CRO | CTX | CAZb | ||
| M-15 | Km (μM) | 7 ± 0. 7 | 11 ± 1 | 30 ± 4 | 22 ± 3 | 14 ± 2 | 15 ± 1 | 27 ± 2 | 972 ± 179 |
| kcat (s−1) | 25 ± 0.4 | 132 ± 11 | 34 ± 2 | 61 ± 2 | 43 ± 2 | 68 ± 1 | 60 ± 2 | ND | |
| kcat/Km (μM−1 s−1) | 3.5 | 12 | 1.1 | 2.8 | 3.1 | 4.5 | 2.2 | ND | |
| M-55 | Km (μM) | 18 ± 2 | 6 ± 0.8 | 15 ± 2 | 15 ± 2 | 10 ± 2 | 8 ± 1 | 23 ± 3 | 592 ± 114 |
| kcat (s−1) | 23 ± 0.6 | 161 ± 9 | 216 ± 8 | 70 ± 3 | 54 ± 3 | 57 ± 3 | 73 ± 4 | ND | |
| kcat/Km (μM−1 s−1) | 1.3 | 26 | 14 | 4.7 | 5.4 | 7.1 | 3.2 | ND | |
| M-132 | Km (μM) | 39 ± 3 | 10 ± 1 | 14 ± 2 | 10 ± 1 | 7 ± 1 | 8 ± 0.4 | 25 ± 4 | 649 ± 98 |
| kcat (s−1) | 42 ± 1 | 242 ± 17 | 226 ± 9 | 64 ± 2 | 43 ± 2 | 74 ± 1 | 83 ± 5 | ND | |
| kcat/Km (μM−1 s−1) | 1.1 | 24 | 16 | 6.4 | 6.1 | 9.3 | 3.3 | ND | |
| M-123 | Km (μM) | 11 ± 0.7 | 9 ± 2 | 16 ± 2 | 19 ± 3 | 8 ± 0.7 | 12 ± 1 | 23 ± 2 | 1,218 ± 254 |
| kcat (s−1) | 428 ± 5 | 183 ± 23 | 214 ± 7 | 116 ± 5 | 69 ± 2 | 124 ± 5 | 95 ± 3 | ND | |
| kcat/Km (μM−1 s−1) | 39 | 20 | 13 | 6.1 | 8.6 | 10 | 4.1 | ND | |
| M-64 | Km (μM) | 17 ± 2 | 10 ± 2 | 25 ± 3 | 31 ± 4 | 16 ± 2 | 10 ± 1 | 24 ± 4 | 1,516 ± 166 |
| kcat (s−1) | 107 ± 3 | 345 ± 34 | 91 ± 3 | 235 ± 12 | 233 ± 14 | 195 ± 7 | 174 ± 9 | ND | |
| kcat/Km (μM−1 s−1) | 6.3 | 35 | 3.6 | 7.6 | 15 | 20 | 7.3 | ND | |
| M-14 | Km (μM) | 72 ± 11 | 11 ± 2 | 64 ± 6 | 22 ± 3 | 14 ± 0.5 | 17 ± 3 | 34 ± 5 | >8,000 |
| kcat (s−1) | 25 ± 2 | 114 ± 12 | 357 ± 18 | 56 ± 3 | 16 ± 0.2 | 42 ± 4 | 37 ± 2 | ND | |
| kcat/Km (μM−1 s−1) | 0.35 | 10 | 5.6 | 2.5 | 1.1 | 2.5 | 1.1 | ND | |
| M-14(D240G) | Km (μM) | 17 ± 2 | 2 ± 0.4 | 68 ± 21 | 28 ± 2 | 13 ± 2 | 14 ± 2 | 30 ± 3 | >8,000 |
| kcat (s−1) | 25 ± 0.8 | 41 ± 3 | 265 ± 57 | 50 ± 2 | 17 ± 1 | 68 ± 4 | 54 ± 3 | ND | |
| kcat/Km (μM−1 s−1) | 1.5 | 21 | 3.9 | 1.8 | 1.3 | 4.9 | 1.8 | ND | |
NCF, nitrocefin.
The values were determined as Ki in the competition assay using nitrocefin as the reporter substrate. ND, the kinetic constants could not be determined due to low activity of the enzymes toward ceftazidime.
TABLE 4.
IC50 and Ki of β-lactamase inhibitorsa
| Enzyme | Clavulanic acid |
Tazobactam |
Sulbactam |
|||
|---|---|---|---|---|---|---|
| IC50 (nM) | Ki (nM) | IC50 (nM) | Ki (nM) | IC50 (nM) | Ki (nM) | |
| CTX-M-15 | 23.2 | 5.40 ± 1.24 | 7.61 | 0.92 ± 0.37 | 50.4 | 6.23 ± 2.51 |
| CTX-M-55 | 13.9 | 2.37 ± 0.80 | 6.88 | 0.86 ± 0.15 | 50.7 | 7.59 ± 1.17 |
| CTX-M-132 | 16.7 | 4.60 ± 0.51 | 3.70 | 1.07 ± 0.16 | 30.8 | 17.3 ± 1.73 |
| CTX-M-123 | 17.6 | 3.37 ± 0.34 | 1.72 | 0.52 ± 0.11 | 59.4 | 11.1 ± 1.55 |
| CTX-M-64 | 22.3 | 5.87 ± 1.28 | 5.75 | 1.44 ± 0.22 | 45.4 | 14.9 ± 1.79 |
| CTX-M-14 | 60.1 | 24.3 ± 2.40 | 7.28 | 1.62 ± 0.25 | 403 | 119 ± 31.3 |
The IC50 and Ki values in the competition experiments were calculated using nitrocefin as the reporter substrate.
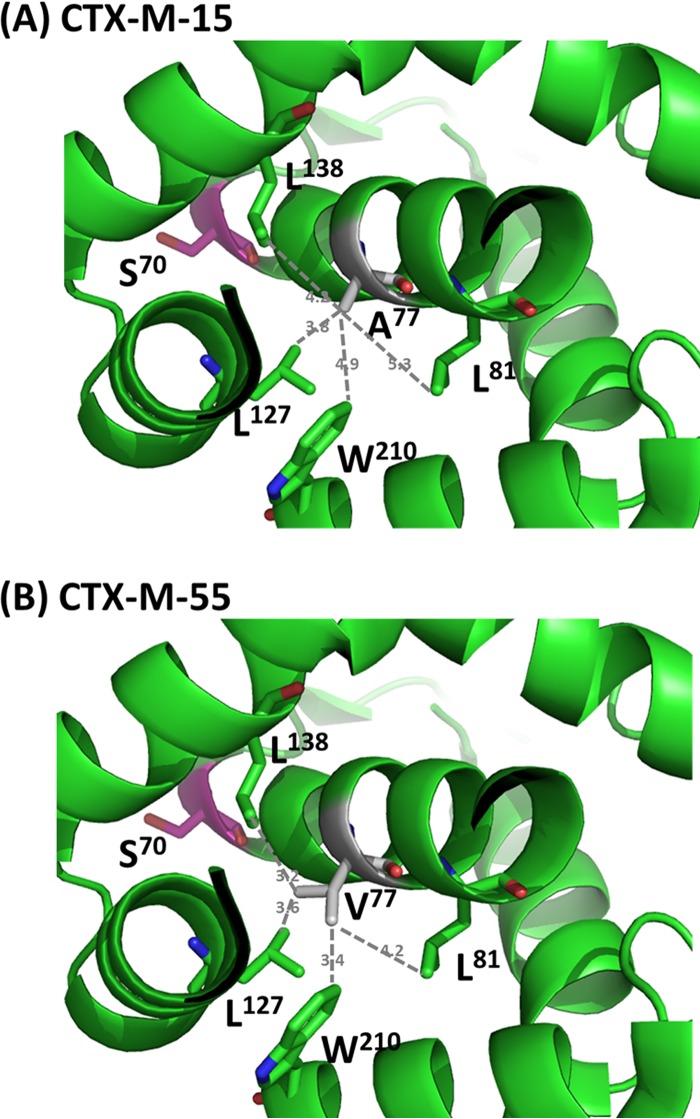
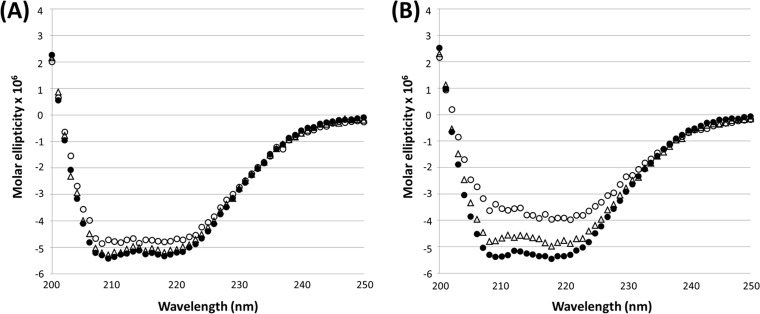
Structural analysis was then performed on these CTX-Ms to investigate the mechanisms responsible for different catalytic activities observed among the enzymes. Structures of CTX-M-14 (1YLT) and CTX-M-15 (4HBT) were adopted from the Protein Data Bank, while structures of other CTX-Ms were modeled by Swiss-Model, with CTX-M-14 and CTX-M-15 as templates. CTX-M-55 harbored only one substitution compared to CTX-M-15. This A77V substitution in CTX-M-55 could be associated with slightly higher catalytic activity of cephalosporins than CTX-M-15, with the highest effect an ∼13-fold increase toward cephalothin (Table 3). Structural analysis showed that both A77 and V77 were located in the center of globular proteins of CTX-M-15 and CTX-M-55. This location suggested that these residues would not contribute to the recognition of substrate but may have an impact on the maintenance of stability of the protein structure. Residue A77 in CTX-M-15 was located in the center of a helix cluster that forms the major part of the enzyme. The loops connecting these helixes contribute to the formation of the active site of CTX-M enzyme. Due to the short side chain of A77, this residue may not be able to form strong hydrophobic interactions with adjacent residues in other helices, limiting its ability to stabilize the helix cluster (Fig. 3A). Upon substitution with V77, however, this residue may be able to form stronger hydrophobic interactions with the key residues, such as L81, L127, L138, and W210, in different helices, reflecting a shorter distance between these residues (Fig. 3B). The structure analysis of A77/V77 of CTX-M-15/CTX-M-55 was supported by results of the thermal stability assay using far-UV CD; CTX-M-15 and CTX-M-55 showed highly similar spectra at 25°C. CTX-M-55 showed similar secondary structure spectra at 25°C and 40°C and showed subtle loss of helicity throughout 200 to 230 nm at 60°C (Fig. 4A). In contrast with CTX-M-55, CTX-M-15 underwent loss of helicity at both 40°C and 60°C, with more loss of helicity at the higher temperature (Fig. 4B). In addition, the activity assay at different temperatures indicated that CTX-M-55 hydrolyzed cefotaxime with slightly reduced activity efficiency, by 1.7-fold, at 40°C and 60°C, whereas CTX-M-15 showed more obviously attenuated kcat/Km, by 3.9- and 6.6-fold at 40°C and 60°C, respectively, compared with that at 25°C. Taken together, these results suggested that CTX-M-55 harbored higher structure stability and was more tolerant to the temperature change (Table 5 and Fig. 4). The higher stability of the protein structure of CTX-M-55, compared to CTX-M-15, may contribute to a more stable active site conformation and, hence, better substrate recognition and catalysis, thereby accounting for the higher catalytic activity of CTX-M-55. Our results were consistent with previous findings showing that A77V played an important role in the phenotype of expanded-spectrum cephalosporin resistance, in which A77V increased resistance to cefotaxime and ceftazidime when associated with P167 and G240 in the background of CTX-M-10 (16). Collectively, these data suggested that, albeit being located distal to the active site, A77V plays an important role on the catalytic activity of CTX-Ms through stabilizing the structure of the enzymes, which has also been reported in other β-lactamases (17).
FIG 3.
Interaction between A77/V77 and other residues in the adjacent helixes of CTX-M-15 and CTX-M-55. (A) A77 in CTX-M-15 interacting with residues L81, L127, L138, and W210: the distances between A77 and L81, L127, L138, and W210 were 5.3 Å, 3.8 Å, 4.2 Å, and 4.9 Å, respectively; (B) V77 in CTX-M-15 interacting with residues L81, L127, L138, and W210: the distances between V77 and L81, L127, L138, and W210 were 4.2 Å, 3.6 Å, 3.2 Å, and 3.4 Å, respectively.
FIG 4.
Determination of secondary structures of CTX-M-55 (A) and CTX-M-15 (B) at 25°C (●), 40°C (Δ), and 60°C (○) by circular dichroism spectrum.
TABLE 5.
Kinetic constants of CTX-M-55 and CTX-M-15 toward cefotaxime at different temperatures
| CTX-M enzyme and kinetic constant | Value at a temp of: |
||
|---|---|---|---|
| 25°C | 40°C | 60°C | |
| CTX-M-55 | |||
| Km (μM) | 23 ± 3 | 45 ± 5 | 53 ± 6 |
| kcat (s−1) | 73 ± 4 | 95 ± 6 | 101 ± 6 |
| kcat/Km (μM−1 s−1) | 3.2 | 2.1 | 1.9 |
| CTX-M-15 | |||
| Km (μM) | 27 ± 2 | 56 ± 10 | 87 ± 12 |
| kcat (s−1) | 60 ± 2 | 32 ± 3 | 39 ± 4 |
| kcat/Km (μM−1 s−1) | 2.2 | 0.6 | 0.4 |
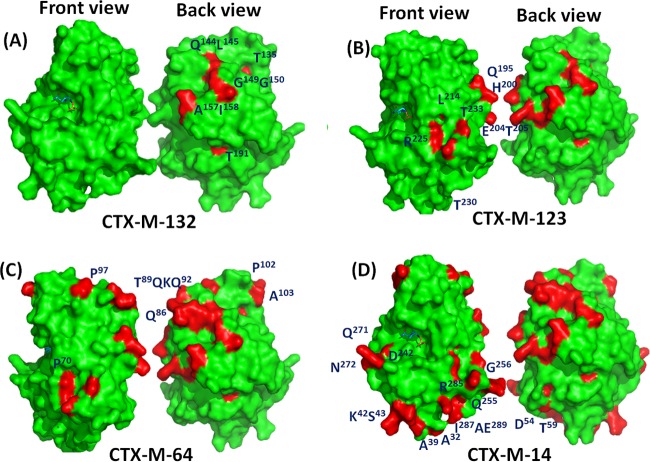
Through sequence alignment of CTX-Ms in Fig. 1, we could see that, if CTX-M-15 was used as parental enzyme template, different hybrids could be formed by introduction of different substitutions from CTX-M-14. Compared to CTX-M-15, CTX-M-132 harbored substitutions at 9 different residues, including S118T, V133T, H141Q, V142L, A146G, S147G, Q154A, L155I, and S182T, which were located at the opposite side of the active site (Fig. 1A and 5A). The enhancement of catalytic activity, determined as kcat/Km, of CTX-M-132 by approximately 2-fold toward nitrocefin, cefuroxime, ceftiofur, and ceftriaxone, and by 13.6-fold for cephalothin, was probably due to the introduction of these substitutions. The localization of these residues on the opposite surface to the active site suggested that surface residues distal to the active site could also contribute to the catalytic activity of CTX-Ms.
FIG 5.
Structural models depicting the locations of divergent residues. Front and back views of the proteins are shown. The front view shows the active site side of protein, while the back view is distal to the active site. The active site is shown as the region with a compound bound. The structure of CTX-M-132, where the labeled residues highlighted in red are different from those in CTX-M-15 (A); the structure of CTX-M-123, where the labeled residues in red are different from those in CTX-M-132 and all the residues highlighted in red are different from those in CTX-M-15 (B); the structure of CTX-M-64, where the labeled residues in red are different from those in CTX-M-123 and all the residues highlighted in red are different from those in CTX-M-15 (C); and the structure of CTX-M-14, where the labeled residues in red are different from those in CTX-M-64 and all the residues highlighted in red are different from those in CTX-M-15 (D).
Further substitution of another set of 9 residues, T118S, N192Q, K197H, D201E, S202T, M211L, Q222R, A227T, and V230T, in CTX-M-132 formed another new hybrid of CTX-M, CTX-M-123 (Fig. 1A and 5B). The catalytic activity of CTX-M-132 and CTX-M-123 toward different β-lactams was similar except toward ampicillin, suggesting these residues showed either a neutral effect or an antagonistic effect between some of them, such as the case of P167S and D240G reported by Novais et al., on the catalytic activity of these two CTX-Ms (16). Nonetheless, it is almost impossible to identify which ones within these 9 residues play important roles on the catalytic activity unless individual mutations are introduced into the background of CTX-M-132. Further substitution of several residues at the N termini, namely, A67P, K83Q, S86T, E87Q, P88K, N89Q, R94P, K99P, S100A, S118T, T227A, and T230V in CTX-M-123, formed CTX-M-64 (Fig. 5C). It may be too speculative to assume these residues play critical roles in enhancing the cephalosporinase activity of CTX-M-64, considering the MIC results were similar for these two CTX-Ms. Nevertheless, the catalytic activity of CTX-M-64 toward several β-lactams, such as nitrocefin, ceftiofur, and ceftriaxone, were indeed higher than those of CTX-M-123, implying that some of these residues, including P67, Q83, T86, Q87, K88, Q89, P94, P99, A100, T118, A227, and V230, which are distal to the active site, may contribute to the catalytic activity of CTX-M-64 although the effects may not be crucial enough to alter the phenotypes of MICs (Tables 2 and 3). Further experiments with the individual mutation for each of the residues will be required to clarify their roles on the catalytic activity of CTX-M-64.
It is well accepted that CTX-M-64 is a hybrid between CTX-M-14 and CTX-M-15 and the closest CTX-M hybrid to CTX-M-14. Sequence analysis of CTX-M-14 and CTX-M-64 showed that these two CTX-Ms differed mainly at both C and N termini. Within these residues, the substitutions of A27S, D28A, E35A, R38K, Q39S, N50D, S56T, I58V, and A62G at the N termini and A227T, V230T, G240D, K254Q, D255G, I260V, P270Q, and K271N at the C termini probably were the most important residues contributing to the dramatically decreased activity of CTX-M-14. Compared to CTX-M-14, these substitutions of CTX-M-64 extended the hydrolytic activities toward ampicillin, nitrocefin, cefuroxime, ceftiofur, ceftriaxone, and cefotaxime but resulted in increased sensitivity to clavulanic acid and sulbactam (Tables 2 to 4). Intriguingly, these residues were mostly located at the lower edge of the CTX-M-64 with the exception of one residue, D240, which is close to the active site of CTX-M-64 (Fig. 5D). D240G has been reported to be associated with a higher MIC toward ceftazidime but reduced resistance to cefotaxime and/or cefepime due to an increased mobility of the B3 β strand and flexibility of the active site pocket, allowing the bulky ceftazidime to more easily accommodate the catalytic cavity (18, 19). To determine whether this residue contributed to the reduction in the catalytic activity observed in CTX-M-14, CTX-M-14(D240G) was generated by site-directed mutagenesis. The MICs of E. coli cells carrying CTX-M-14(D240G) were similar to that of CTX-M-14 toward all of the β-lactams tested (Table 2). The kinetic constants of CTX-M-14(D240G) showed that this substitution showed a limited effect on cephalosporin hydrolysis, with 2-fold differences, while slightly increased the kcat/Km toward ampicillin and nitrocefin by 4.3- and 2.1-fold, respectively, in our hands (Table 3). These results were somehow inconsistent with the finding that the single substitution of D240G in CTX-M-14 to become CTX-M-27 conferred a higher MIC toward ceftazidime, yet this mutation also decreased the hydrolytic activity against other good substrates, such as cefuroxime and cefotaxime, compared to CTX-M-14 in a study by Bonnet et al. (20). The inconsistency may be due to differences in the methods and conditions used to determine the kinetic parameters in our approach and theirs, which used a computerized microacidimetric method (20).
Comparative analyses of these data suggested that, by acquisition of the middle part of CTX-M-14, the catalytic activity of CTX-M-15 could be enhanced through formation of highly efficient hybrids, such as CTX-M-132, CTX-M-123, and CTX-M-64. However, further acquisition of N and C termini of CTX-M-14 would dramatically decrease the catalytic activity of CTX-M-15. From the evolution point of view, it is likely that the formation of hybrids is through homologous recombination between CTX-M-14 and CTX-M-15, driven by the evolutionary pressure favoring selection of expanded-spectrum cephalosporins. Epidemiological findings that the hybrid CTX-Ms are more commonly reported from E. coli strains in regions where the use of expanded-spectrum cephalosporins is commonplace than in regions where expanded-spectrum cephalosporins are used less, support this hypothesis (21, 22).
ACKNOWLEDGMENTS
We thank Yohei Doi for useful discussions and critical review of the manuscript.
This work was supported by the Chinese National Key Basic Research and Development (973) Program (2013CB127200), the Program for Changjiang Scholars and Innovative Research Team in University (IRT13063), and the National Natural Science Foundation of China (U1031004).
We declare no conflicts of interest.
REFERENCES
- 1.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type beta-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian GB, Huang YM, Fang ZL, Qing Y, Zhang XF, Huang X. 2014. CTX-M-137, a hybrid of CTX-M-14-like and CTX-M-15-like beta-lactamases identified in an Escherichia coli clinical isolate. J Antimicrob Chemother 69:2081–2085. doi: 10.1093/jac/dku126. [DOI] [PubMed] [Google Scholar]
- 4.He D, Partridge SR, Shen J, Zeng Z, Liu L, Rao L, Lv L, Liu JH. 2013. CTX-M-123, a novel hybrid of the CTX-M-1 and CTX-M-9 group beta-lactamases recovered from Escherichia coli isolates in China. Antimicrob Agents Chemother 57:4068–4071. doi: 10.1128/AAC.00541-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagano Y, Nagano N, Wachino J, Ishikawa K, Arakawa Y. 2009. Novel chimeric beta-lactamase CTX-M-64, a hybrid of CTX-M-15-like and CTX-M-14 beta-lactamases, found in a Shigella sonnei strain resistant to various oxyimino-cephalosporins, including ceftazidime. Antimicrob Agents Chemother 53:69–74. doi: 10.1128/AAC.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantón R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front Microbiol 3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv L, Partridge SR, He L, Zeng Z, He D, Ye J, Liu JH. 2013. Genetic characterization of IncI2 plasmids carrying blaCTX-M-55 spreading in both pets and food animals in China. Antimicrob Agents Chemother 57:2824–2827. doi: 10.1128/AAC.02155-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirel L, Gniadkowski M, Nordmann P. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum beta-lactamase CTX-M-15 and of its structurally related beta-lactamase CTX-M-3. J Antimicrob Chemother 50:1031–1034. doi: 10.1093/jac/dkf240. [DOI] [PubMed] [Google Scholar]
- 9.Ma L, Ishii Y, Chang FY, Yamaguchi K, Ho M, Siu LK. 2002. CTX-M-14, a plasmid-mediated CTX-M-type extended-spectrum beta-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother 46:1985–1988. doi: 10.1128/AAC.46.6.1985-1988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, Liu BG, Liu JH, Pan YS, Yuan L, Hu GZ. 2012. Phenotypic and molecular characterization of CTX-M-14 extended-spectrum beta-lactamase and plasmid-mediated ACT-like AmpC beta-lactamase produced by Klebsiella pneumoniae isolates from chickens in Henan Province, China. Genet Mol Res 11:3357–3364. doi: 10.4238/2012.September.24.1. [DOI] [PubMed] [Google Scholar]
- 11.Zheng H, Zeng Z, Chen S, Liu Y, Yao Q, Deng Y, Chen X, Lv L, Zhuo C, Chen Z, Liu JH. 2012. Prevalence and characterisation of CTX-M beta-lactamases amongst Escherichia coli isolates from healthy food animals in China. Int J Antimicrob Agents 39:305–310. doi: 10.1016/j.ijantimicag.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Faheem M, Rehman MT, Danishuddin M, Khan AU. 2013. Biochemical characterization of CTX-M-15 from Enterobacter cloacae and designing a novel non-beta-lactam beta-lactamase inhibitor. PLoS One 8:e56926. doi: 10.1371/journal.pone.0056926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laraki N, Franceschini N, Rossolini GM, Santucci P, Meunier C, de Pauw E, Amicosante G, Frere JM, Galleni M. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-beta-lactamase IMP-1 produced by Escherichia coli. Antimicrob Agents Chemother 43:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii Y, Galleni M, Ma L, Frere JM, Yamaguchi K. 2007. Biochemical characterisation of the CTX-M-14 beta-lactamase. Int J Antimicrob Agents 29:159–164. doi: 10.1016/j.ijantimicag.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Novais A, Canton R, Coque TM, Moya A, Baquero F, Galan JC. 2008. Mutational events in cefotaximase extended-spectrum beta-lactamases of the CTX-M-1 cluster involved in ceftazidime resistance. Antimicrob Agents Chemother 52:2377–2382. doi: 10.1128/AAC.01658-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgianni L, Vandenameele J, Matagne A, Bini L, Bonomo RA, Frere JM, Rossolini GM, Docquier JD. 2010. Mutational analysis of VIM-2 reveals an essential determinant for metallo-beta-lactamase stability and folding. Antimicrob Agents Chemother 54:3197–3204. doi: 10.1128/AAC.01336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Delmas J, Sirot J, Shoichet B, Bonnet R. 2005. Atomic resolution structures of CTX-M beta-lactamases: extended-spectrum activities from increased mobility and decreased stability. J Mol Biol 348:349–362. doi: 10.1016/j.jmb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Delmas J, Chen Y, Prati F, Robin F, Shoichet BK, Bonnet R. 2008. Structure and dynamics of CTX-M enzymes reveal insights into substrate accommodation by extended-spectrum beta-lactamases. J Mol Biol 375:192–201. doi: 10.1016/j.jmb.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet R, Recule C, Baraduc R, Chanal C, Sirot D, De Champs C, Sirot J. 2003. Effect of D240G substitution in a novel ESBL CTX-M-27. J Antimicrob Chemother 52:29–35. doi: 10.1093/jac/dkg256. [DOI] [PubMed] [Google Scholar]
- 21.Rao L, Lv L, Zeng Z, Chen S, He D, Chen X, Wu C, Wang Y, Yang T, Wu P, Liu Y, Liu JH. 2014. Increasing prevalence of extended-spectrum cephalosporin-resistant Escherichia coli in food animals and the diversity of CTX-M genotypes during 2003 to 2012. Vet Microbiol 172:534–541. doi: 10.1016/j.vetmic.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Xia S, Fan X, Huang Z, Xia L, Xiao M, Chen R, Xu Y, Zhuo C. 2014. Dominance of CTX-M-type extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from patients with community-onset and hospital-onset infection in China. PLoS One 9:e100707. doi: 10.1371/journal.pone.0100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambler RP, Coulson AF, Frere JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem J 276:269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]