FIG 1.
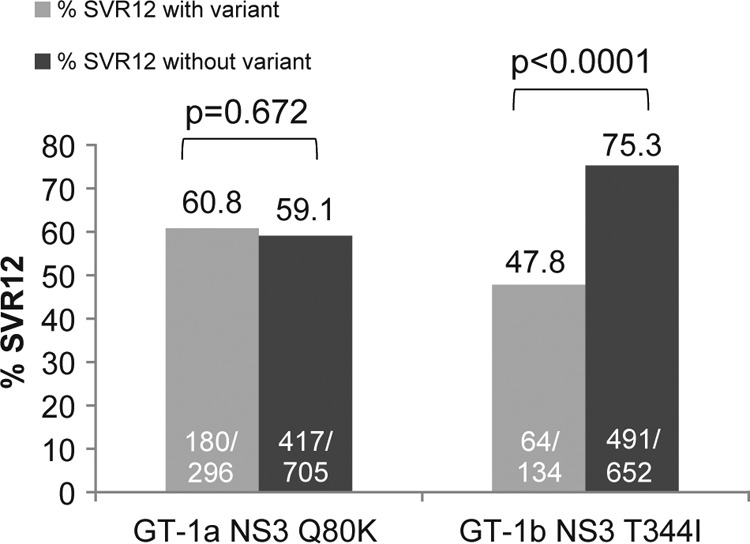
Impact of baseline NS3 polymorphisms on response to faldaprevir plus PR in phase 3 studies. Data pooled from phase 3 studies (STARTVerso1 to -4) including faldaprevir plus PR-treated patients with SVR12 and those without SVR12 for any reason (breakthrough during faldaprevir plus PR treatment or PR only or relapse or experiencing virologic failure for other reasons). “With variant” includes only the single amino acid variant of interest and does not include the wild type, other variants, or mixtures of the variant of interest with wild-type or other amino acids. “Without variant” includes the wild type and all other amino acid variants or mixtures detected. P values were determined using Fisher's exact test. GT, genotype; PR, pegylated interferon/ribavirin; SVR12, sustained virologic response at 12 weeks posttreatment.
