Figure 1.
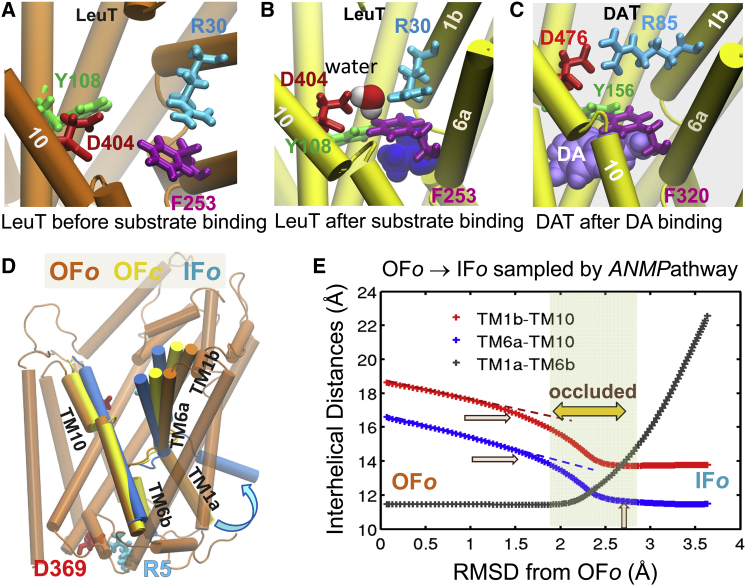
Local and global structural changes captured by full-atomic and coarse-grained structure-based computations. (A and B) Closure of the extracellular (EC) gate after substrate binding, observed in MD simulations of leucine transporter (LeuT) (23). LeuT is known to transport alanine (most efficiently), leucine, and other amino acids. Panel A displays its substrate binding site in the outward-facing, open (OFo) crystal structure (24), before substrate binding. The oppositely charged residues R30 and D404, originally far apart (A), closely interact in upon substrate binding (leucine, blue space filling) (B), as illustrated for the LeuT in substrate-bound outward-facing closed (OFc) state (25). Likewise, the two aromatic residues, Y108-F253 closely associate to form another layer further consolidating the EC gate (23). (C) MD simulations of a LeuT structural homolog, human dopamine transporter (hDAT, modeled after the OFo dDAT structure (26)) show local changes in conformation upon dopamine (DA) (light violet space filling) binding (27), which closely resemble those stabilized in substrate-bound LeuT. R85-D476 and Y156-F320 serve as the EC gates in this case. Transmembrane (TM) helical segments TM1a-b, TM6a-b, and TM10 that line the binding cavity, exhibit significant structural rearrangements. (D) Global structural differences between LeuT in OFo state before substrate binding (dark orange), OFc state after substrate binding (yellow), and inward-facing open (IFo) state (blue) after substrate release to the intracellular (IC) medium (24) (E) Global structural changes observed in ENM-based simulations of OFo ↔ IFo transition (28). TM1b-TM10, TM6a-TM10, and TM1a-TM6b center-of-mass (COM) distances serve as metrics for probing the extent of reconfiguration. The passage over an occluded state where the transporter is closed to both EC and IC media, consistent with experimental data (25), is highlighted.