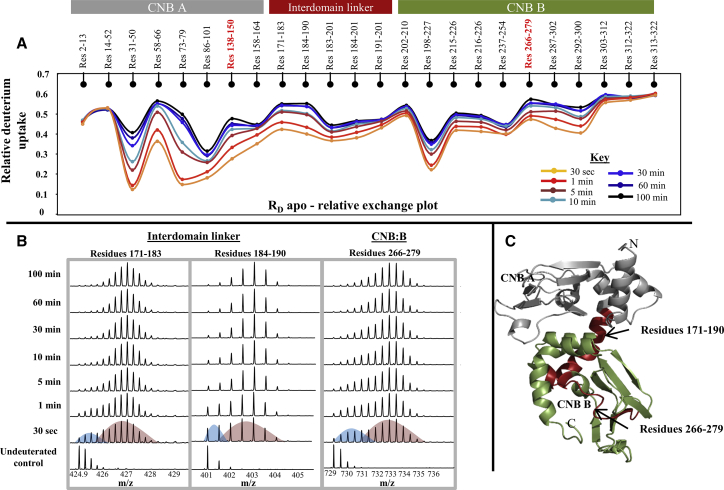
Figure 2.
Apo RD is a dynamic protein with regions that undergo slow structural transitions/local unfolding. (A) The relative deuterium uptake value (calculated as the ratio of average deuterium ions exchanging to the maximum exchangeable amides; y axis) for each pepsin digest fragment from the N- to C-terminus (x axis) of RD is plotted in a relative exchange plot. The relative exchange plot provides a snapshot of the overall dynamics of RD for each deuterium labeling time point as depicted in the key. Peptides spanning the cAMP binding pocket in CNB A and B are highlighted (red). Plots were generated using the software DYNAMX. (B) Stacked mass spectra of the three peptides exhibiting bimodal distributions are shown. Mass spectra are stacked in order of increasing deuterium labeling time (y axis) as shown. Colored curves are used to represent lower-exchanging (blue curve) and higher-exchanging (red curve) distributions in the 30-s labeling time of spectra. (C) Peptides showing bimodal characteristics are mapped (red) onto the modeled structure of RD. (Gray) CNB:A (residues 1–180) and (green) CNB:B (residues 181–327); the N- and C-termini of the protein and CNB pocket in A and B domains are labeled.