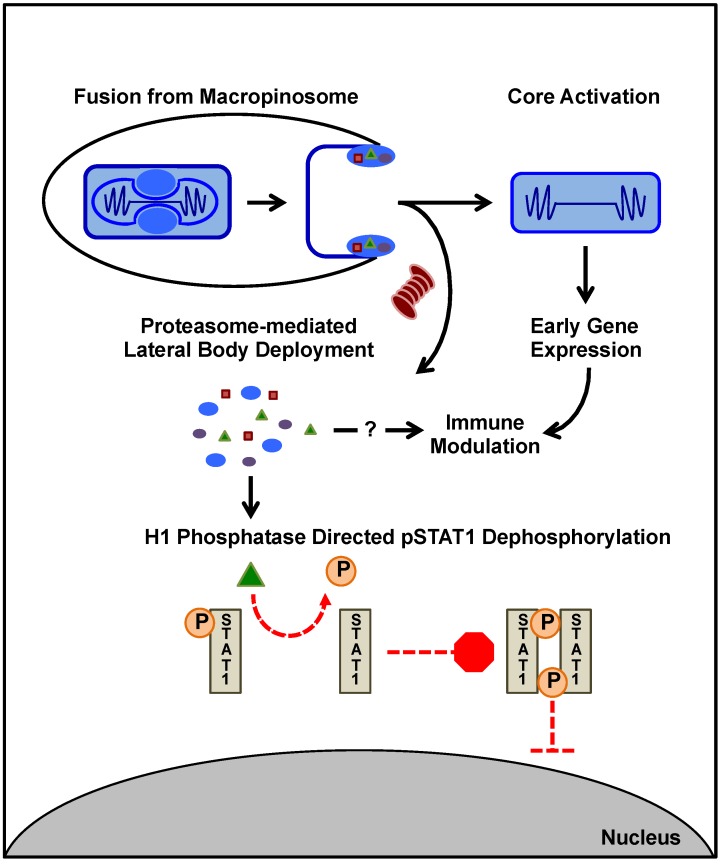
Figure 2.
VACV LBs as Immunomodulatory Delivery Packets. After internalisation via macropinocytosis VACV particles undergo fusion with the limiting membrane of the macropinosome releasing the viral core into the cytoplasm. The released viral cores are “activated” as indicated by morphological changes and the initiation of early gene expression from within. Upon fusion, the LBs detach from the core and remain associated with the viral membrane. Once exposed to the cytoplasm, LBs are rapidly disassembled, with the major LB structural protein, F17, undergoing proteasome dependent degradation. Disassembly of the LB appears to facilitate release of other LB proteins and, in the case of the viral dual specificity phosphatase H1, is required for their action. Release of H1 from LBs, serves to shunt cellular antiviral transcription prior to the expression of early viral genes. To do this, H1 dephosphorylates phospho-STAT1 preventing its homodimerisation and nuclear translocation. To date only three LB components F17, H1, and a viral disulfide oxidoreductase G4 have been identified.