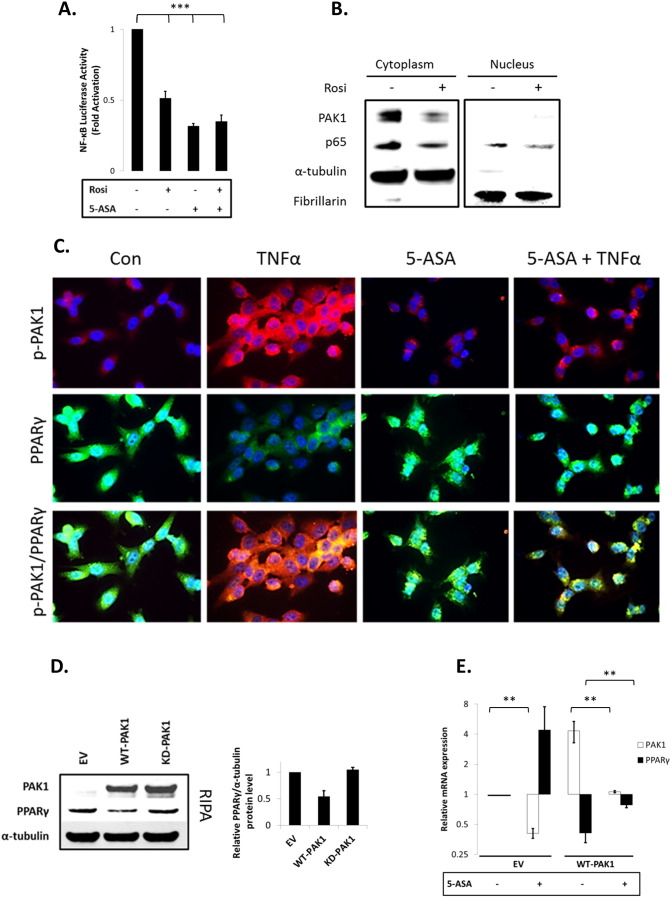
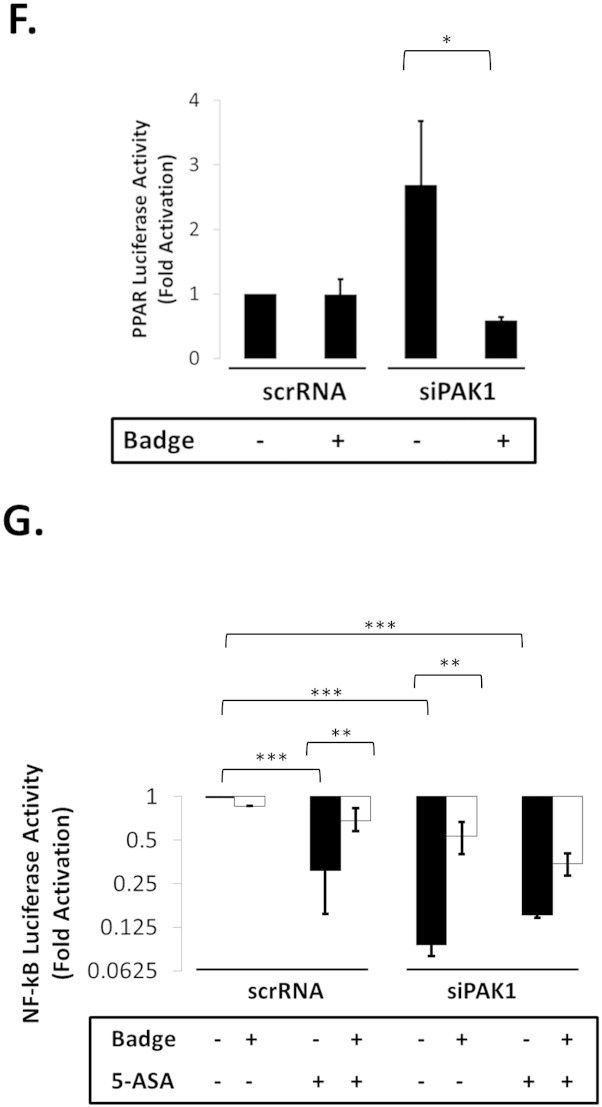
Fig. 4.
(A–G). PAK1 modulates a PPARγ/p65 cascade. (A) HCEC-1CT cells were transfected with a NF-kB luciferase reporter and treated with 1 μM Rosi ± 20 mM 5-ASA (24 h). Rosi and 5-ASA blocked NF-kB transcriptional activity ANOVA, Tukey HSD; ***p < 0.001. (B) Rosi treated cells were fractionated and analyzed by WB for PAK1 and p65. Rosi blocked PAK1 and p65 expression. (C) IF of p-PAK1 and PPARγ upon TNFα (30 min) with or without 20 mM 5-ASA pretreatment (24 h). TNFα resulted in p-PAK1 activation and PPARγ nuclear export and downregulation which was blocked by 5-ASA. (D) The effect of EV, WT-PAK1, and KD-PAK1 overexpression on PPARγ was analyzed by WB. α-Tubulin was used as a loading control. Densitometry of PPARγ levels normalized to α-tubulin, data are mean and SD from 2 independent experiments. (E) RTPCR of PAK1 and PPARγ after EV or WT-PAK1 overexpression with or without 5-ASA treatment (24 h). PAK1 overexpression blocked PPARγ at the mRNA level, an effect that was recovered by 5-ASA. Data are representative of 3 independent experiments (t-test, 2 tailed; **p < 0.01). (F) PPAR luciferase reporter assay in PAK1 knock down cells using scrRNA or siPAK1. siPAK1 activated PPAR transcription, and this effect was recovered upon 0.1 μM BADGE pretreatment (12 h), ANOVA, Tukey HSD; *p < 0.05. (G) NF-kB luciferase assay in PAK1 knock down cells using scrRNA or siPAK1. Cells were pretreated with BADGE (12 h) with or without 5-ASA (24 h). BADGE recovered the effect of 5-ASA or siPAK1 on NF-kB. All data are representative of 3 independent experiments ANOVA, Tukey HSD; **p < 0.01, ***p < 0.001.