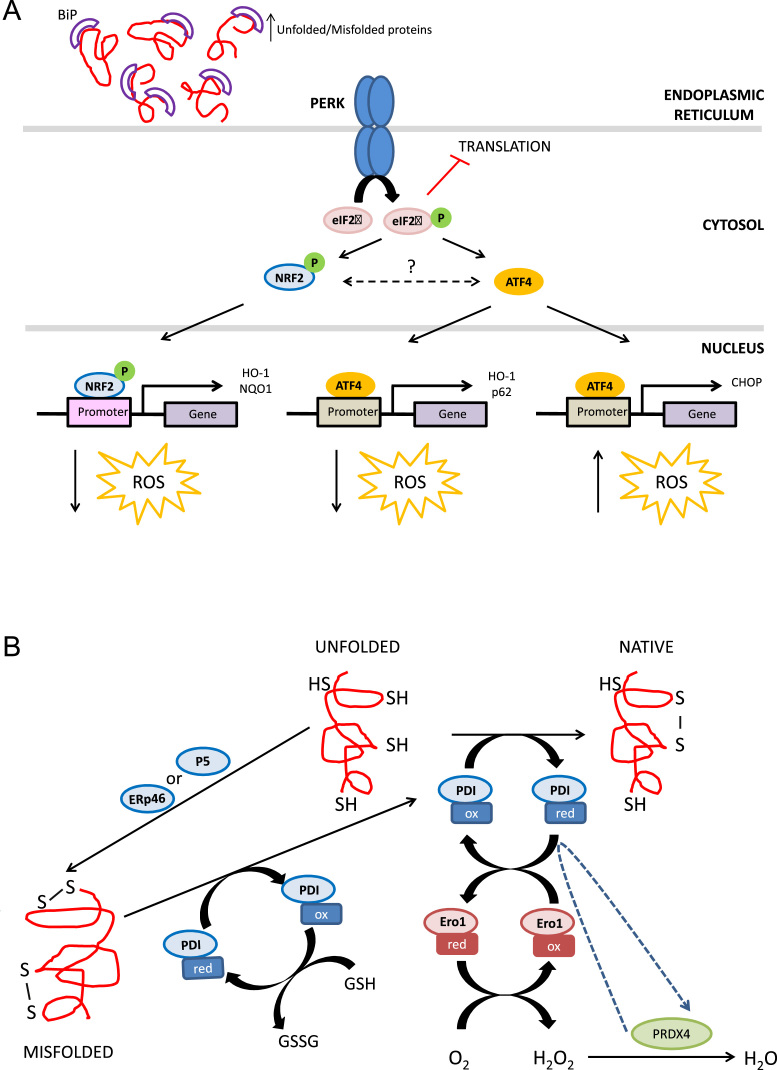
Fig. 6.
Impact of protein folding on oxidative stress and the UPR. A, Oxidative environment in the ER is crucial for correct protein folding. The highly oxidative environment in the ER (1:1–1:3 GSH/GSSH ratio compared to the 30:1–100:1 in the cytosol) is required for protein folding; ROS are formed as byproducts of normal protein folding and as a consequence of incorrect disulfide bond formation. Protein disulfide isomerases (PDIs) catalize disulfide bond formation in nascent proteins, a process necessary for correct protein folding. Two Cys in the active site of PDI accept two electrons from the Cys of the folding polypeptide. PDIs are then oxidized by oxidoreductin 1 (ERO1) proteins that subsequently transfer electrons to oxygen to produce H2O2. The generated H2O2 is metabolized into H2O by peroxiredoxin IV. Moreover, improperly paired disulfide bonds can be reduced by PDIs in parallel with glutathione oxidation. ROS produced during protein folding and the consumption of GSH upon the reduction of improperly paired disulfide bonds may shift the redox balance in the ER, favoring the accumulation of misfolded/unfolded proteins and activating the UPR. B, The UPR modulates redoxstasis. One of the three UPR arms activated upon ER stress is PERK signaling. PERK phosphorylates NRF2, promoting its nuclear translocation and induction of the antioxidant response. PERK signaling also involves phosphorylation of eIF2α and a transient inhibition of protein translation. However, selective translation of the transcription factor ATF4 is induced under these circumstances. ATF4 promotes an antioxidant response through the expression of target genes such as HO-1. Although a crosstalk between ATF4 and NRF2 has been described, the underlying mechanisms remain unclear. ATF4-induced CHOP expression results in the expression of Ero1α, an enzyme that causes Ca2+ leakage from the ER, activating CaMKII in the cytosol, which in turn induces NOX2 and causes oxidative stress. Moreover, CHOP has been implicated in the transcriptional repression of Bcl2 and transactivation of BIM and PUMA that leads to enhanced oxidant injury and apoptosis.