Abstract
Gastrin-releasing peptide receptor (GRPR) is ectopically expressed in over 60% of colon cancers. GRPR expression has been correlated with increased colon cancer cell migration. However, the signaling pathway by which GRPR activation leads to increased cancer cell migration is not well understood. We set out to molecularly dissect the GRPR signaling pathways that control colon cancer cell migration through regulation of small GTPase RhoA. Our results show that GRP stimulation activates RhoA predominantly through G13 heterotrimeric G-protein signaling. We also demonstrate that postsynaptic density 95/disk-large/ZO-1 (PDZ)-RhoGEF (PRG), a member of regulator of G-protein signaling (RGS)-homology domain (RH) containing guanine nucleotide exchange factors (RH-RhoGEFs), is the predominant activator of RhoA downstream of GRPR. We found that PRG is required for GRP-stimulated colon cancer cell migration, through activation of RhoA–Rho-associated kinase (ROCK) signaling axis. In addition, PRG-RhoA-ROCK pathway also contributes to cyclo-oxygenase isoform 2 (Cox-2) expression. Increased Cox-2 expression is correlated with increased production of prostaglandin-E2 (PGE2), and Cox-2-PGE2 signaling contributes to total GRPR-mediated cancer cell migration. Our analysis reveals that PRG is overexpressed in colon cancer cell lines. Overall, our results have uncovered a key mechanism for GRPR-regulated colon cancer cell migration through the Gα13-PRG-RhoA-ROCK pathway.
Introduction
Gastrin-releasing peptide (GRP) receptor (GRPR) has been found to be ectopically expressed or overexpressed in small-cell lung cancer (SCLC), breast cancer, prostate cancer, and colon cancer (Jensen et al., 2008). In colon cancer, several studies support the role of GRPR in increasing tumor cell proliferation (Radulovic et al., 1991; Frucht et al., 1992), and morphogenic transformation leading to increased tumor cell differentiation (Carroll et al., 1999). GRPR has also been shown to stimulate colon cancer cell motility (Glover et al., 2005). However, the molecular mechanisms by which GRPR activation leads to colon cancer cell migration are not well understood.
GRPR is a seven-transmembrane G-protein coupled receptor (GPCR) that couples to members of the Gq/11 and G12/13 families of heterotrimeric G-proteins (Jensen et al., 2008). GRPR-mediated cancer cell proliferation is thought to be primarily regulated through activation of Gαq canonical signaling pathway (Cuttitta et al., 1985; Rozengurt, 1998; Jensen et al., 2008). In comparison with Gαq signaling, relatively little is known about Gα12/13-mediated pathways downstream of GRPR and their contributions to colon cancer progression. Receptors coupled to Gα12/13 are known to activate small GTPase RhoA that controls cell migration (Wang et al., 2004; Mikelis et al., 2013). This is accomplished by direct interaction of activated Gα13 with the family of guanine nucleotide exchange factors (GEFs) for RhoA known as regulator of G-protein signaling (RGS)-homology domain (RH) containing guanine nucleotide exchange factors (RH-RhoGEFs). The RH-RhoGEF subfamily consists of p115RhoGEF (p115), postsynaptic density 95/disk-large/ZO-1 (PDZ)-RhoGEF (PRG), and leukemia-associated RhoGEF (LARG) (Hart et al., 1996,1998; Kozasa et al., 1998; Fukuhara et al., 1999, 2000; Tanabe et al., 2004). GTP-bound Gα13 interacts with the RH domain of these large multidomain-containing GEFs. This interaction stimulates their GEF activity leading to activation of RhoA (Hart et al., 1998; Kozasa et al., 1998; Chen et al., 2008). Thus, RH-RhoGEFs are primary candidates that may link GRPR stimulation to RhoA activation. However, the specific GEF(s) mediating RhoA activation downstream of GRPR in colon cancer cells has not been established.
Activation of RhoA is known to contribute to tumorigenesis by playing a role in cellular transformation, proliferation, migration, and invasion (Sahai and Marshall, 2002). Several studies have shown that RhoA is overexpressed and highly activated in many solid tumors, including colon cancer (Fritz et al., 1999; Karlsson et al., 2009). Increased RhoA activation and signaling through its downstream effectors, such as Rho-associated kinase (ROCK), contributes to cancer progression. Activation of the RhoA-ROCK signaling axis initiates cytoskeletal changes that are essential for cancer cell motility and invasion, initiates gene transcription, and promotes cancer cell proliferation (Yagi et al., 2011; Vigil et al., 2012; Zhang et al., 2013). It is currently unknown if GRPR-mediated RhoA-ROCK signaling plays a role in regulation of colon cancer cell migration.
In this report, we have elucidated the signaling pathway downstream of GRPR that is critical for colon cancer cell migration. We have determined that PRG is the dominant GEF mediating RhoA activation downstream of GRPR in colon cancer cells. We have demonstrated that GRPR signaling through Gα13-PRG-RhoA axis is critical for colon cancer cell migration. Furthermore, we also report that GRP-stimulated PRG-RhoA-ROCK signaling regulates cyclo-oxygenase-2 (Cox-2) expression, and find that Cox-2 activity contributes to the overall GRP-stimulated colon cancer cell migration.
Materials and Methods
Human gastrin-releasing peptide was purchased from Sigma-Aldrich (St. Louis, MO), celecoxib and Y-27632 [(1R,4r)-4-((R)-1-aminoethyl)-N-(pyridin-4-yl)cyclohexanecarboxamide] were purchased from Tocris (Bristol, UK), Primary Human Colonic Epithelial Cells (HCoEpiC) lysate was purchased from ScienCell Research Laboratories (Carlsbad, CA). Normal human distal colon mucosal sample (male sample) was a gift from Pradeep Dudeja, University of Illinois at Chicago.
Cell Culture and Transfection.
Caco-2 and HT-29 cells (gift from Richard Benya, Loyola Medicine Chicago, IL) were maintained in base medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, glutamine, and sodium pyruvate, along with Ham’s F12 medium with glutamine. Caco-2 cells were cultured in base medium supplemented with 20% fetal bovine serum (FBS), and HT-29 supplemented with 10% FBS. Human embryonic kidney (HEK)293T cells were cultured in DMEM supplemented with 10% FBS. All cell culture reagents were purchased from Invitrogen/Life Technologies (Grand Island, NY). Caco-2 cells were transfected with Silencer Select PRG small-interfering (si)RNA (s19005) with the sequence 5′-GAGAUGAAACGGUCUCGAAtt-3′, Silencer Select PRG siRNA (s19006) with sequence 5′-GCGAAACCCUAUCCUCAAtt-3′, and Silencer Select Negative control no. 1 siRNA (AM4611) purchased from Invitrogen. Gα13 knockdown was achieved by siGENOME Human GNA13 siRNA–SMARTpool (M-009948-00-0005) consisting of four GNA13–specific siRNA sequences 5′-GAGAUAAGAUGAUGUCGUU-3′, 5′-CCAAGGAGAUCGACAAAUG-3′, 5′-GAGAGAAGCUUCAUAUUCC-3′, 5′-GAAGAUCGACUGACCAAUC-3′ purchased from GE Healthcare/Dharmacon (Lafayette, CO). Small-interfering (siRNA) transfection was done with Lipofectamine RNAiMAX per manufacturer’s protocol from Invitrogen. All experiments utilizing cells with siRNA knockdown were conducted 48 hours after siRNA transfection, and postserum starvation for 16 hours. All cells were between 50 to 70% confluent when experiments were carried out.
Western Blotting.
Cells were lysed in 20 mM HEPES (pH 7.6), 1% Triton-X-100, 150 mM NaCl, 5 mM MgCl2, 2 mM Na3VO4, 1 mM β-glycerophosphate, aprotinin (16 μg/ml), and leupeptin (3.2 μg/ml). Cell lysates were then clarified by centrifugation at 14,500 RPM for 10 minutes at 4°C. Protein concentration of the lysate was then verified with Bio-Rad DC purchased from Bio-Rad (Hercules, CA). SDS-PAGE sample buffer was then added to the lysate and the samples were boiled for 3 minutes and resolved by SDS-PAGE. Protein was then transferred to nitrocellulose membrane (GE Healthcare) and blocked with 5% milk in Tris-buffered saline and Tween 20 (T-BST) for 1 hour at room temperature. Membranes were then incubated with one of the following antibodies at 4°C: anti-RhoA monoclonal, anti-PDZ-RhoGEF polyclonal, anti-LARG polyclonal (gift from Takao Hamakubo University of Tokyo, 1:1000), anti-p115 polyclonal, anti-GAPDH monoclonal, anti–Green Fluorescent Protein (GFP) polyclonal, anti-Cox-2 polyclonal from Cell Signaling Technology (Danvers, MA), anti-Gα13 polyclonal, anti-Gα13 polyclonal B860 (1:1000) (Singer et al., 1994), anti-Gαq/11 polyclonal, anti-Gα12 polyclonal, and anti-beta-actin monoclonal from Sigma-Aldrich. All other antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). Membranes were then probed with horseradish peroxidase–conjugated secondary antibodies from Amersham (GE Healthcare Life Sciences, Piscataway, NJ). Western blots were developed with SuperSignal West Pico Chemiluminescent Substrate from Thermo Scientific (Rockford, IL). Densitometry was performed with ImageJ software.
RhoA GTPase Pull-Down Assay.
Rho activity in cultured cells was assessed utilizing the manufacturer’s (Cytoskeleton, Inc., Denver, CO) protocol. Briefly, colon cancer cells were serum-starved for 16 hours. After stimulation, the cells were lysed at 4°C in buffer containing 50 mM Tris-HCl pH (7.5), 300 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 2 mM Na3VO4, aprotinin (16 μg/ml), and leupeptin (3.2 μg/ml). The lysates were then incubated with glutathione S-transferase (GST)-Rho-GTP binding domain of Rhotekin bound to glutathione-Sepharose beads purchased from Cytoskeleton, Inc. (Denver, CO). The samples were washed three times with wash buffer (per manufacturer’s instructions), and then resuspended in SDS-PAGE sample buffer. Samples were then analyzed by Western blot with monoclonal RhoA antibody.
Purification of GST-RhoAG17A Recombinant Protein.
Plasmid construct for the prokaryotic expression of GST-RhoAG17A was provided by K. Burridge (University of North Carolina). Purification of GST-RhoAG17A was carried out as previously described (Garcia-Mata et al., 2006). Briefly, expression of GST-RhoAG17A in BL21- CodonPlus (DE3)-RP purchased from Stratagene/Agilent Technologies (Santa Clara, CA) was induced with 200 μM isopropyl-β-d-thiogalactoside (IPTG) for 16 hours at 18°C. Bacterial cells were then lysed with 20 mM HEPES (pH 7.6), 1% Triton-X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT), aprotinin (16 μg/ml), and leupeptin (3.2 μg/ml). Protein was purified by incubating glutathione-Sepharose 4B beads, purchased from GE Healthcare, at 4°C for 45 minutes. Sepharose beads were then washed with lysis buffer twice and twice with 20 mM HEPES (pH 7.6), 150 mM NaCl, 5 mM MgCl2, and 1 mM dithiothreitol. Protein concentration was estimated with Coomassie Plus protein reagent (Thermo Scientific). The beads were then aliquoted and snap frozen with liquid nitrogen and stored at –80°C.
GST-RhoAG17A Pull-Down Assay.
Activation of RH-RhoGEFs was monitored with GST-RhoAG17A pull-down assay as previously described (Garcia-Mata et al., 2006). Briefly, Caco-2 cells were stimulated with 100 nM GRP for indicated time(s), after which cells were then lysed with 20 mM HEPES (pH 7.6), 1% Triton-X-100, 150 mM NaCl, 5 mM MgCl2, 2 mM Na3VO4, aprotinin (16 μg/ml), and leupeptin (3.2 μg/ml) at 4°C. Protein concentration of lysates was verified with Bio-Rad DC (BioRad), and equal protein and volume of lysate was incubated with 30 μg of purified GST-RhoAG17A–bound glutathione-Sepharose beads for 45 minutes at 4°C. Samples were then washed three times with lysis buffer without 1% Triton-X-100, and the beads were resuspended in SDS-PAGE sample buffer. Samples were then analyzed by Western blot with RH-RhoGEF–specific antibodies.
Generation of Lentivirus.
The cDNAs encoding GFP, GFP-RH-GRK2 (1-178aa of bovine GRK2), and GFP-RH-RGS3 (378-519aa of human RGS3) were subcloned under EF-1a promoter of lentivirus transfer vector pLVTH (Cambridge, MA). Lentiviruses were generated as previously described (Wiznerowicz and Trono, 2003). In short, pLVTH (transfer vector) encoding GFP, GFP-RH-RGS3, or GFP-RH-GRK2 were transfected to HEK293T cells together with pMD2.G (envelope) and pCMVDR8.74 (packaging vector) by the calcium phosphate precipitation method. Lentivirus produced (packaged) by HEK293T cells were harvested from cell medium 48 hours later.
Coimmunoprecipitation Assay.
HEK293T cells were infected with lentivirus for GFP, GFP-conjugated RH-RGS3, or RH-GRK2. After 48 hours the cells were harvested on ice and the lysates were used for pulldown assay with anti-Gαq/11 antibody (Santa Cruz Biotechnology) as previously described (Tesmer et al., 2005).
Intracellular Calcium Measurement.
Agonist-induced intracellular calcium mobilization was performed in serum-free condition with GFP, RH-RGS3, and RH-GRK2 expressing Caco-2 cells with GFP-Certified FluoForte calcium assay kit for microplates according to manufacturer’s protocol (Enzo Life Sciences, Farmingdale, NY). Intracellular calcium mobilization was monitored by Molecular Devices (Sunnyvale, CA) FlexStation System. Fluorescence was monitored at Ex = 530 nm/Em = 570 nm. Data obtained as ratio of fold increase after stimulation over basal.
Prostaglandin E2 Enzyme-Linked Immunosorbent Assay.
Cell culture media was collected at 4°C after incubating with GRP (100 nM) for indicated time(s). Cell culture media was then centrifuged at 8000 RPM to clear cellular debris. Culture media was then either assayed or stored at –80°C for no longer than 7 days. Concentration of prostaglandin E2 (PGE2) in the culture media was obtained using PGE2 Express EIA Kit following manufacturer’s protocol (Cayman Chemical, Ann Arbor, MI).
Cell Migration Assays.
Cells were serum-starved for 16 hours prior to the assay. Caco-2 and HT-29 cells were plated on the upper chamber of six-well 8.0-μm-pore polycarbonate membrane insert (Corning, Tewksbury, MA) at a density of 5 × 105 cells/well. The inserts were placed in 1% FBS containing media with or without GRP (100 nM) added to the lower chamber. The plate was then placed in the incubator at 37°C supplemented with 5% CO2. Cells were allowed to migrate for 8 hours, after which the cells on the top of the chamber were mechanically removed and the inserts were washed with PBS. The cells were fixed with 4% paraformaldehyde for 10 minutes (Electron Microscopy Sciences, Hatfield, PA) and stained with 2% crystal violet (Sigma-Aldrich) for 5 minutes. Migrated cells on the lower chamber were visualized with a microscope and counted.
Data Analysis and Statistics.
Statistical and graphical analysis was conducted with GraphPad Prism 5 (La Jolla, CA). Data are represented as mean ± S.E.M of at least n = 3 independent experiments. Statistical analysis was performed with one-way analysis of variance followed by Bonferroni’s multiple comparison test.
Results
GRP Stimulation Increases RhoA Activation in Colon Cancer Cells.
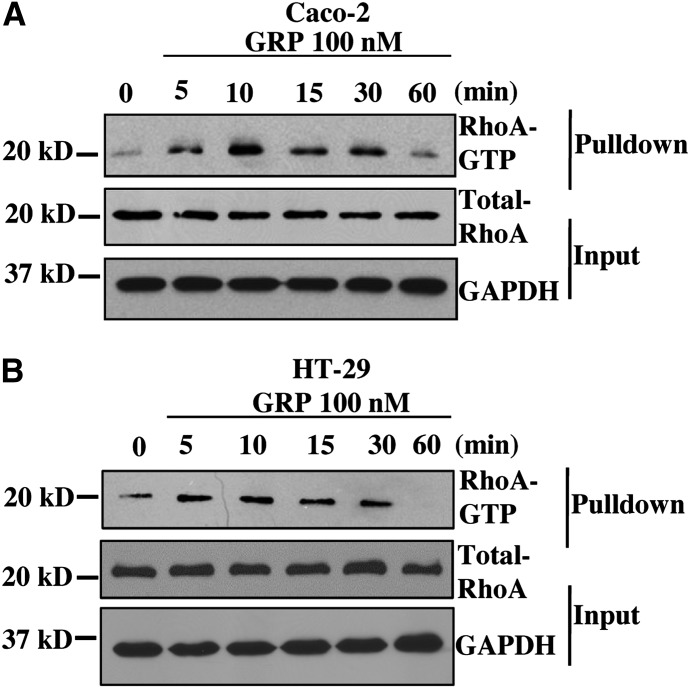
GRPR expression is absent in normal colonic epithelial cells (Carroll et al., 1999). However, its ectopic expression on colon cancer cells contributes to tumorigenesis by stimulating cell proliferation and migration (Frucht et al., 1992; Jensen et al., 2008). Previous studies indicate that GRPR can promote tumorigenicity through activation of the small GTPase RhoA in prostate cancer (Zheng et al., 2006). However, the role of RhoA signaling downstream of GRPR in colon cancer has not been well studied. Thus, we first sought to determine whether activation of GRPR leads to activation of RhoA in colon cancer cells. As a model we used Caco-2 and HT-29 colon cancer cell lines, which express functional GRP receptor and form moderately well-differentiated adenocarcinoma in nude mice (Carroll et al., 2000). To determine RhoA activation, we conducted a time-course experiment, stimulating Caco-2 and HT-29 cells in serum free conditions with a concentration of GRP (100 nM) that has been used for previous colon cancer studies (Ferris et al., 1997; Glover et al., 2005).The level of RhoA activation was assessed using RhoA pulldown assay (Ren and Schwartz, 2000) (Fig. 1, A and B). Stimulating colon cancer cells with GRP increased the fraction of RhoA in the active GTP-bound state. The activation of RhoA reaches maximum at about 10 minutes and decreases over time out to 60 minutes after GRP addition in both Caco-2 and HT-29 cells. These data indicate that GRPR activation on colon cancer cells initiates signaling pathway(s) that leads to RhoA activation.
Fig. 1.
GRP stimulation results in RhoA activation in colon cancer cell lines. Time-course of RhoA activation in colon cancer cell lines in response to GRP stimulation. Caco-2 (A) and HT-29 cells (B) serum-starved overnight and then incubated with GRP for indicated time(s). Cell lysates were used for GST-RBD pulldown (see Materials and Methods). The precipitate and lysate samples were then used for Western blot to detect RhoA and GAPDH. GAPDH is used as loading control. Shown are representative images from three independent experiments. RBD, Rho binding domain.
Gα13 Is the Principal Mediator of RhoA Activation Downstream of GRPR.
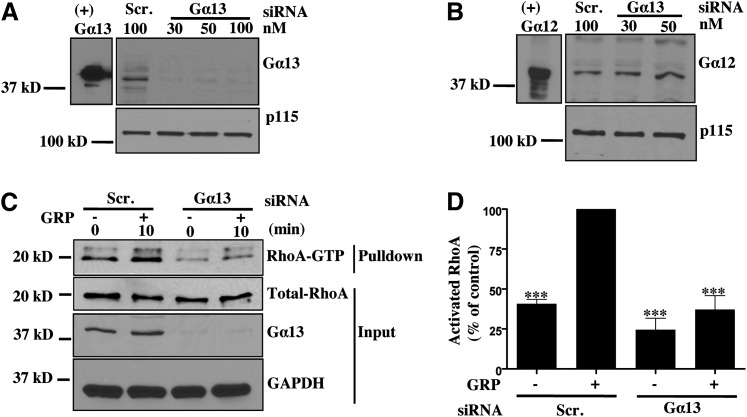
GRPR signaling is in part conducted through activation of the alpha subunits of Gq and G12/13 heterotrimeric G-proteins in colon cancers (Jensen et al., 2008). However, the contribution of each G-protein to GRPR-mediated activation of RhoA in colon cancer cells has not been established. To address this question, we used siRNA to downregulate endogenous Gα13 expression in Caco-2 cells. Gα13 siRNA efficiently and specifically decreased Gα13 expression in Caco-2 cells (Fig. 2A) without affecting expression of its close structural homolog Gα12 (Fig. 2B). Downregulation of Gα13 expression led to a significant decrease in GRP-stimulated RhoA activation, indicating that in Caco-2 cells RhoA activation predominantly occurs through Gα13 (Fig. 2, C and D).
Fig. 2.
Gα13 predominantly mediates RhoA activation downstream of GRPR in Caco-2 cells. (A, B) Caco-2 cells transfected with scrambled or Gα13-SMARTpool siRNA for 48 hours to obtain Gα13-specific knockdown without affecting Gα12 expression. (+) Gα12/13 lanes contain purified recombinant Gα12 or Gα13 subunits used as positive control. (C) Caco-2 cells were serum-starved overnight and then stimulated with GRP for 10 minutes and subsequently used for GST-RBD pulldown (see Materials and Methods). Precipitate and lysate samples were then immunoblotted to detect RhoA, Gα13, and GAPDH. GAPDH was used as loading control. (D) Statistical densitometric analysis of n = 4. Shown are mean values ± S.E.M.; ***P < 0.001. RBD, Rho binding domain.
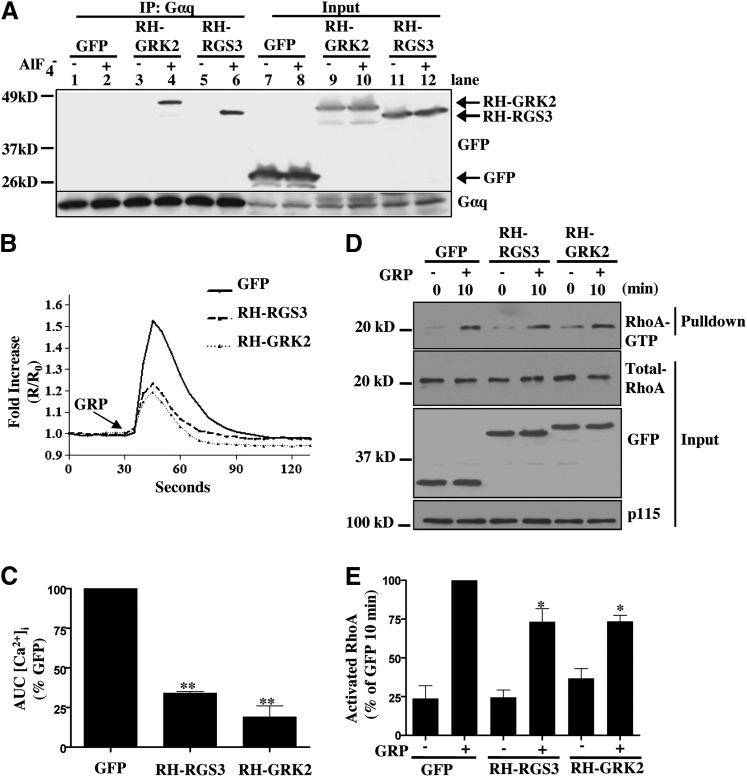
Gαq can also activate RhoA through direct interaction with RhoGEFs such as p63RhoGEF, Trio, and Kalirin (Aittaleb et al., 2010). To determine the role of Gαq in mediating RhoA activation in Caco-2 cells, we transduced Caco-2 cells with lentivirus expressing GFP fused to the RH domain of RGS3 or G-protein coupled receptor kinase 2 (GRK2). Both of these proteins specifically bind to activated Gαq and inhibit Gαq-mediated signaling (Carman et al., 1999; Scheschonka et al., 2000). As shown in Fig. 3A, both RH-RGS3 and RH-GRK2 coimmunoprecipitated with AlF4-activated endogenous Gαq. Caco-2 cells expressing RH-RGS3 and RH-GRK2 also had a defect in GRP-stimulated rise in intracellular Ca2+, a known indicator of GRPR-mediated Gαq signaling, in comparison with GFP-expressing cells (Fig. 3, B and C). These cells were then stimulated with GRP, and lysates were used for RhoA pulldown. Expression of RH-RGS3 or RH-GRK2 led to a small reduction in RhoA activation in response to GRP stimulation (Fig. 3, D and E). This indicates that Gαq makes a minor contribution to total RhoA activation downstream of GRPR, and that Gα13 is the predominant mediator of RhoA signaling.
Fig. 3.
Gαq makes a small contribution to total RhoA activation downstream of GRPR. (A) HEK293T cells were infected with lentivirus for GFP, GFP-conjugated RH-RGS3, or RH-GRK2. After 48 hours, cells were harvested and lysed in the buffer containing either GDP or GDP-AlF4–. Lysates were subjected to Western blotting to confirm protein expression of Gαq/11 (lower panel, lanes 7–12) and GFP, RH-RGS3, or RH-GRK2 (upper panel, lanes 7–12). Immunoprecipitation was carried out using anti-Gαq/11 antibody (lanes 1–6). RH-GRK2 (lane 4, upper) and RH-RGS3 (lane 6, upper) were coprecipitated with endogenous Gαq/11 activated by GDP-AlF4–. (B,C) Caco-2 cells stably expressing GFP, RH-RGS3, and RH-GRK2 were used to monitor GRP-induced calcium mobilization (see Materials and Methods). Shown are representative traces of at least three independent experiments, from which area under the curve (AUC) was quantitated and plotted (**P < 0.01). (D) Caco-2 cells stably expressing GFP, RH-RGS3, and RH-GRK2 were serum-starved overnight and then stimulated with GRP for 10 minutes. Cell lysates were then used for GST-RBD pulldown (see Materials and Methods). Precipitate and lysate samples were then immunoblotted to detect RhoA, GFP, and p115. Expression of GFP, RH-RGS3, RH-GRK2 in the lysate was confirmed with anti-GFP antibody. p115 was used as loading control. (E) Statistical densitometric analysis of n = 5. Shown are mean values ± S.E.M.; *P < 0.05, ***P < 0.001. RBD, Rho binding domain.
PRG Is the Primary RH-RhoGEF Activated Downstream of GRPR.
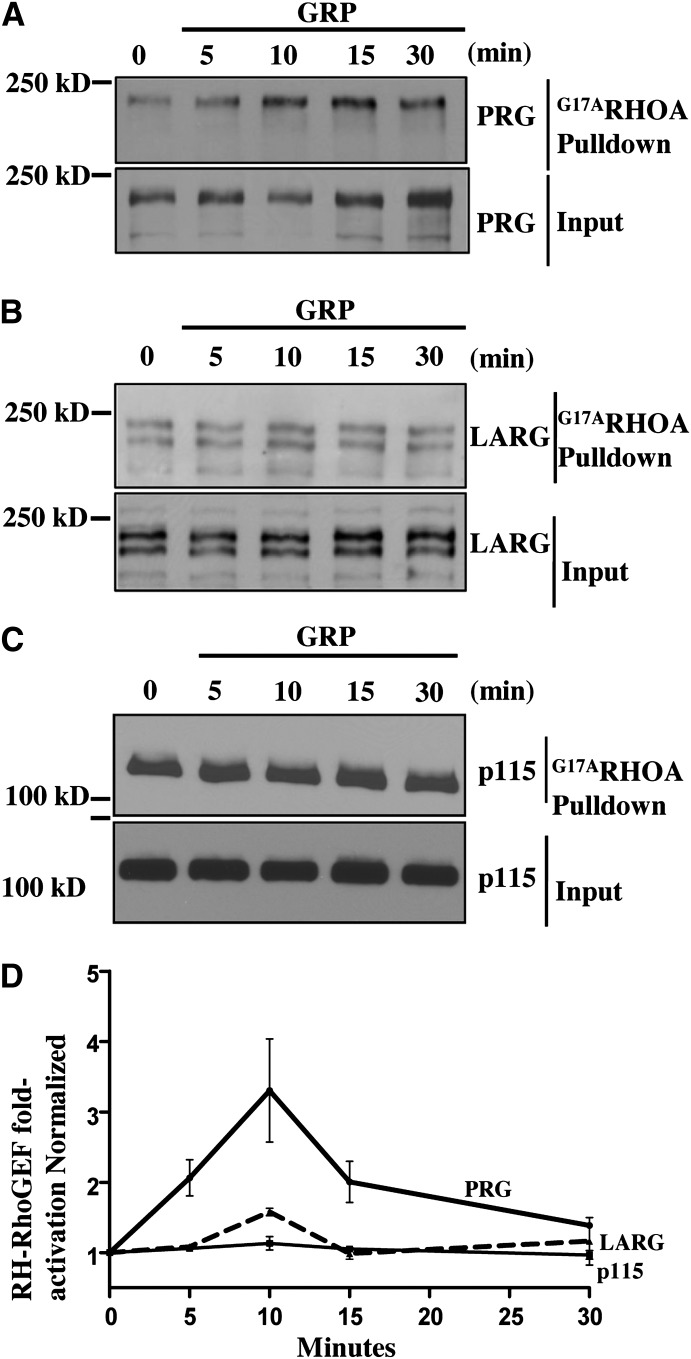
GPCRs coupled to G12/13 family of heterotrimeric G-proteins can initiate RhoA signaling by physically interacting with and activating RH-RhoGEFs. Previous studies have suggested that GPCRs coupled to Gα12/13 use distinct RH-RhoGEFs to activate RhoA signaling (Wang et al., 2004). In Caco-2 cells all three RH-RhoGEF family members (p115, PRG, and LARG) are expressed. (Fig. 4, A–C). Thus, to identify which RH-RhoGEF(s) are activated in response to GRP stimulation, we used GST-RhoAG17A fusion protein as an affinity reagent to isoate activated GEFs for RhoA. The glycine-to-alanine mutation in the recombinant RhoA protein mimics the nucleotide-free state of RhoA which binds with high affinity to activated GEFs (Garcia-Mata et al., 2006). Employing this biochemical approach, we isolated activated GEFs from Caco-2 cells treated with GRP in a time course experiment. Our data reveal that GRP stimulation resulted in strong activation of PRG as indicated by increased PRG pulldown throughout our time-course (Fig. 4A). The maximum activity was detected at 10 minutes after addition of GRP, consistent with the peak of RhoA activity that we observed. In contrast, GRP treatment of Caco-2 cells did not affect activation of LARG and p115 (Fig. 4, B–D). Thus, our data demonstrates that GRPR stimulation predominantly activates PRG in colon cancer cells.
Fig. 4.
PRG is predominantly activated downstream of GRPR. (A–C) Caco-2 cells were serum-starved overnight and then stimulated with GRP for indicated times. The lysates were subsequently used for RhoAG17Apulldown, where GST-RhoAG17A protein is used to pulldown activated RhoGEFs from total cell lysate (see Materials and Methods). Precipitates and the lysate samples were then immunoblotted for PRG, LARG, and p115RhoGEF. Shown are representative images of three independent experiments. (D) Densitometric analysis of activation states of three RH-RhoGEFs normalized to endogenous RH-RhoGEF levels and expressed as fold activation over 0-minute time-point. Shown are mean values ± S.E.M.
PRG Is The Primary Activator of RhoA Downstream of GRPR.
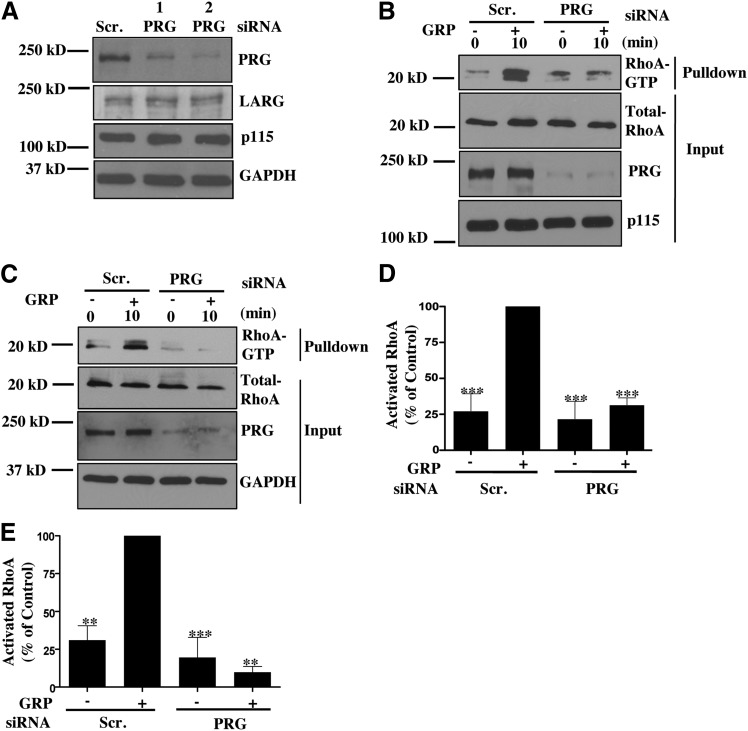
Our RhoAG17A pulldown data reveal that GRP stimulation predominantly activates PRG. This suggests that PRG should be the predominant activator of RhoA downstream of GRPR in colon cancer cells. To confirm this hypothesis, we downregulated expression of PRG using two different siRNA reagents (Fig. 5A). Importantly, treatment with these siRNAs did not affect expression of the two related RH-RhoGEFs, LARG and p115 (Fig. 5A). We then performed a RhoA pulldown with siRNA-transfected cells to determine the role of PRG in RhoA activation in response to GRP stimulation. As shown in Figs. 5, B–E, PRG knockdown significantly decreased GRP-stimulated RhoA activation in Caco-2 and HT-29 cells. Similar decrease in RhoA activation was also observed with the PRG siRNA-2 reagent. Importantly, the decrease in RhoA activation was similar to the effect achieved by downregulation of Gα13 (Fig. 2A). Thus, these data suggest that GRP-mediated RhoA activation in colon cancer cells occurs primarily through the Gα13-PRG signaling axis.
Fig. 5.
PRG knockdown decreases RhoA activation upon GRP stimulation. (A) PRG knockdown was confirmed by utilizing two different siRNA. Specific knockdown of PRG was verified by immunoblotting for LARG and p115RhoGEF. (B,C) Caco-2 (B) or HT-29 (C) cells were transfected with scrambled or PRG siRNA for 48 hours. Cells were serum-starved overnight and the following day were stimulated with GRP for 10 minutes. Cell lysates were then used for GST-RBD pulldown (see Materials and Methods) and samples were then subjected to Western blotting. (D,E) Statistical densitometric analysis of at least three independent experiments. Shown are mean values ± S.E.M. for Caco-2 (D) and HT-29 (E) cells; **P < 0.01, ***P < 0.001.
The PRG-RhoA-ROCK Axis Mediates GRP-Stimulated Colon Cancer Cell Migration.
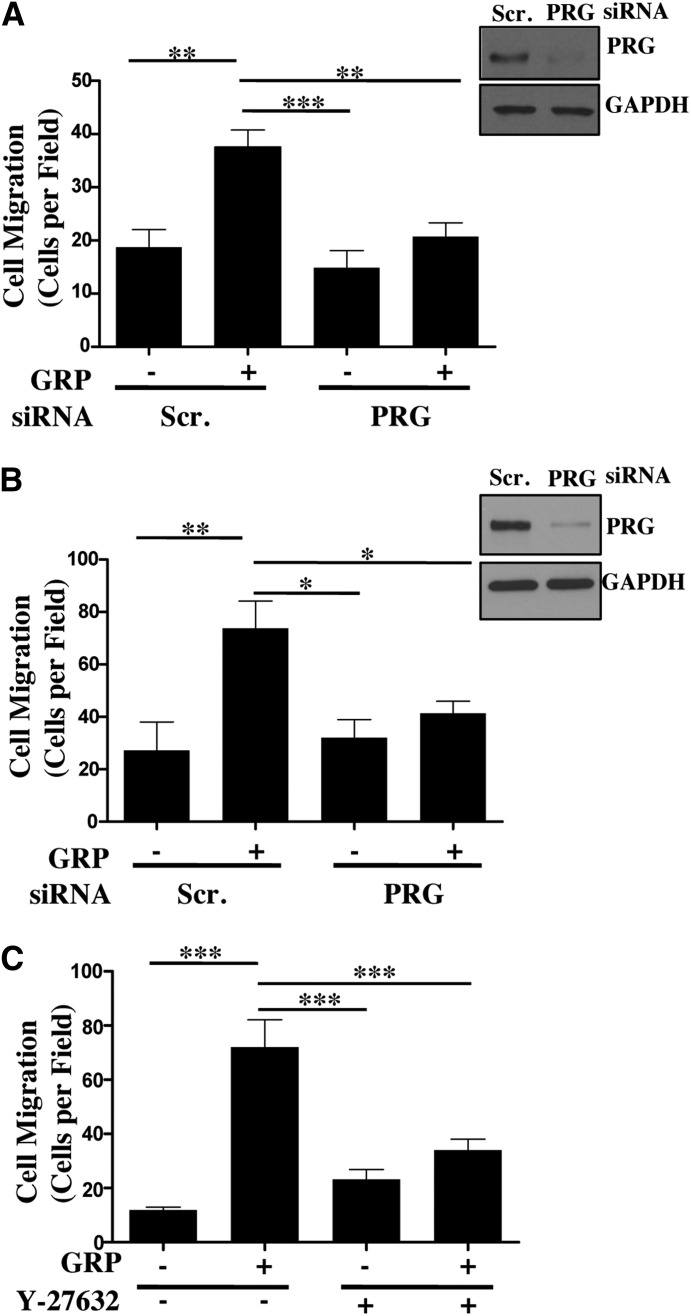
Cancer cell motility is an essential process of cancer progression and invasion. RhoA is known to play a critical role in regulation of focal adhesions and stress fiber formation leading to cell migration (Ridley and Hall, 1992; Hopkins et al., 2007; Yagi et al., 2011). RhoA has been shown to be overexpressed in colon cancers (Fritz et al., 1999). Here we have shown that PRG is the predominant activator of RhoA downstream of GRPR in colon cancer cells. This evidence suggests that PRG should regulate colon cancer cell migration downstream of GRPR. To test this hypothesis we conducted a Transwell cell migration assay using Caco-2 and HT-29 cells transfected with scrambled siRNA or PRG siRNA. As shown in Fig. 6, A and B, PRG knockdown resulted in a dramatic reduction in GRP-stimulated colon cancer cell migration almost to a level equivalent to unstimulated scrambled siRNA-treated cells. This demonstrates that PRG is a critical mediator of colon cancer cell migration downstream of GRPR.
Fig. 6.
PRG-RhoA-ROCK axis mediates GRP-stimulated colon cancer cell migration. (A,B) Caco-2 (A) or HT-29 (B) cells transfected with scrambled or PRG siRNA for 48 hours. The transfected cells were serum-starved overnight and plated on the top chamber of Transwell insert at 5 × 105 cells/well. The inserts were placed in 1% FBS containing media with or without 100 nM GRP (see Materials and Methods). Representative images of PRG knockdown in Caco-2 and HT-29 cells. Statistical analysis of cell migration of n = 3 repeated in duplicates. Shown are mean values ± S.E.M.; (*P < 0.05, **P < 0.01, ***P < 0.001). (C) Caco-2 cells were plated on the top of the Transwell inserts at 5 × 105 cells/well in media with or without GRP along with Y-27632 (20 μM) (see Materials and Methods). Statistical analysis of cell migration of n = 3 repeated in duplicates. Shown are mean values ± S.E.M.; **P < 0.01, ***P < 0.001.
ROCK is one of the key downstream effectors of RhoA and is known to contribute to RhoA-mediated regulation of cancer cell migration and invasion (Vishnubhotla et al., 2007; Yagi et al., 2011). Therefore, to determine the role of ROCK in GRP-stimulated colon cancer cell migration, we conducted the Transwell assay with Caco-2 cells treated with or without ROCK inhibitor Y-27632 (20 μM) in the presence or absence of GRP. As shown in Fig. 6C, ROCK inhibition arrested GRP-stimulated Caco-2 cell migration. In agreement with previous reports, unstimulated Caco-2 cells treated with Y-27632 did have a slight increase in basal cell migration in comparison with cells with no treatment (Hopkins et al., 2007; Makrodouli et al., 2011). Nonetheless, our results indicate that ROCK is required for efficient GRP-stimulated colon cancer cell migration. Overall, our data demonstrate that in colon cancer cells, GRP-stimulated migration is regulated via the PRG-RhoA-ROCK pathway.
PRG Contributes to Cox-2 Expression and PGE2 Production.
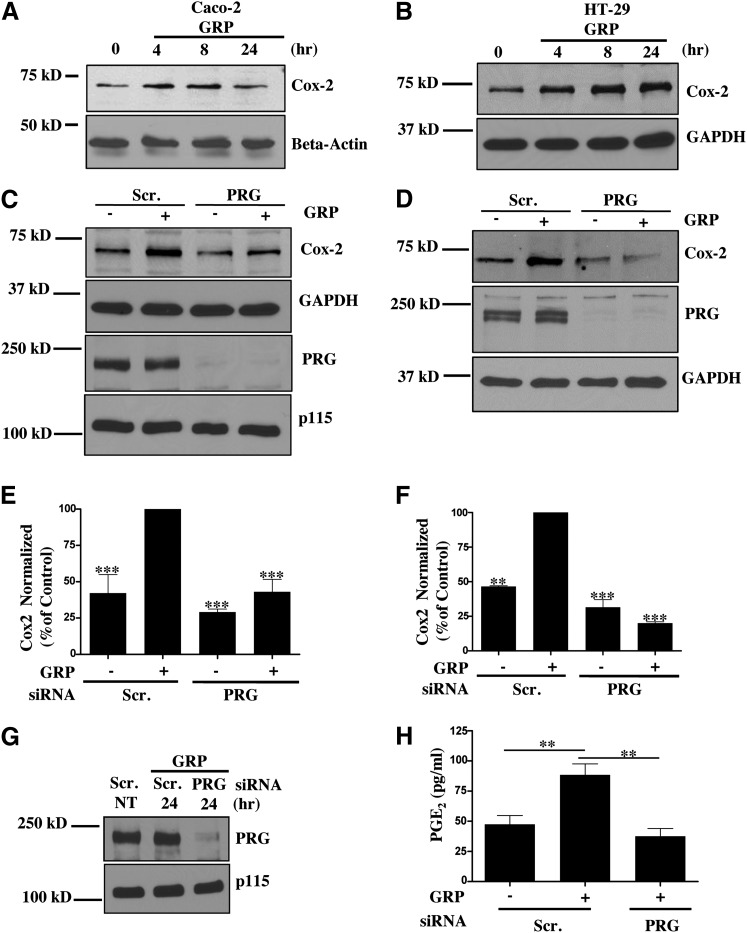
Cox-2 plays a critical role in colon cancer development and progression. Studies have shown that 85% of colon cancers have increased Cox-2 expression (Wang and Dubois, 2010). GRPR signaling has been implicated in regulation of Cox-2 expression in variety of tissues via different mechanisms (Slice et al., 1999; Corral et al., 2007). However, the role of Gα13-mediated signaling pathway in regulation of Cox-2 expression in colon cancer cells has not been elucidated. Hence, we set out to determine if Gα13 signaling downstream of GRPR, specifically the PRG-RhoA-ROCK axis, plays a role in Cox-2 expression. As shown in Fig. 7, A and B, Cox-2 expression is increased upon GRP stimulation in both Caco-2 and HT-29 cells. Cox-2 expression is increased in these cancer cells at 4 and 8 hours after GRP addition. Next, we looked at the role of PRG in regulation of Cox-2 expression in Caco-2 and HT-29 cells downstream of GRPR. Downregulation of PRG expression using siRNA reduced Cox-2 expression after 8 hours of treatment with GRP (Fig. 7, C–F). It is well known that Cox-2 expression drives colon cancer progression through the production of PGE2 (Mutoh et al., 2002; Wang and Dubois, 2010). In fact, PGE2 is the predominant prostaglandin found in colon cancer (Rigas et al., 1993). So we next examined if the decrease in Cox-2 expression in PRG siRNA-transfected cells is associated with a decrease in PGE2 production. We used enzyme-linked immunosorbent assay to quantitate PGE2 concentration in the media of scrambled or PRG siRNA–transfected cells stimulated with GRP. As shown in Figs. 7, G and H, PRG knockdown inhibited GRP-induced production of PGE2 in comparison with scrambled siRNA-treated cells stimulated with GRP. These data show that GRPR-Gα13 signaling through PRG regulates Cox-2 expression and PGE2 production.
Fig. 7.
PRG contributes to Cox-2 expression downstream of GRPR. (A,B) Time course of Cox-2 expression. Caco-2 (A) or HT-29 (B) cells were stimulated with GRP for the indicated time(s). Cox-2 expression was determined by Western blot utilizing Cox-2-specific antibody. Beta-actin and GAPDH used as loading control. (C,D) Cox-2 expression in Caco-2 (C) or HT-29 (D) cells transfected with scrambled or PRG siRNA. The cells were incubated with GRP for 8 hours. Cox-2 expression and PRG knock down was verified by Western Blot. (E,F) Statistical densitometric analysis of Cox-2 expression from n = 3 for Caco-2 (E) and HT-29 (F) cells. Shown are mean values ± S.E.M.; ***P < 0.001. (G,H) PGE2 production in Caco-2 cells transfected with scrambled or PRG siRNA. (G) PRG knock down was confirmed with Western blot. (H) Caco2 cells serum-starved overnight and stimulated with GRP for 24 hours. Cell media for each condition was harvested and analyzed for PGE2 concentration by enzyme-linked immunosorbent assay (ELISA) (see Materials and Methods). Statistical analysis of n = 4. Shown are mean values ± S.E.M.; **P < 0.01.
Rho-ROCK–Mediated Regulation of Cox-2-PGE2 Production Contributes to Overall GRP-Stimulated Cancer Cell Migration.
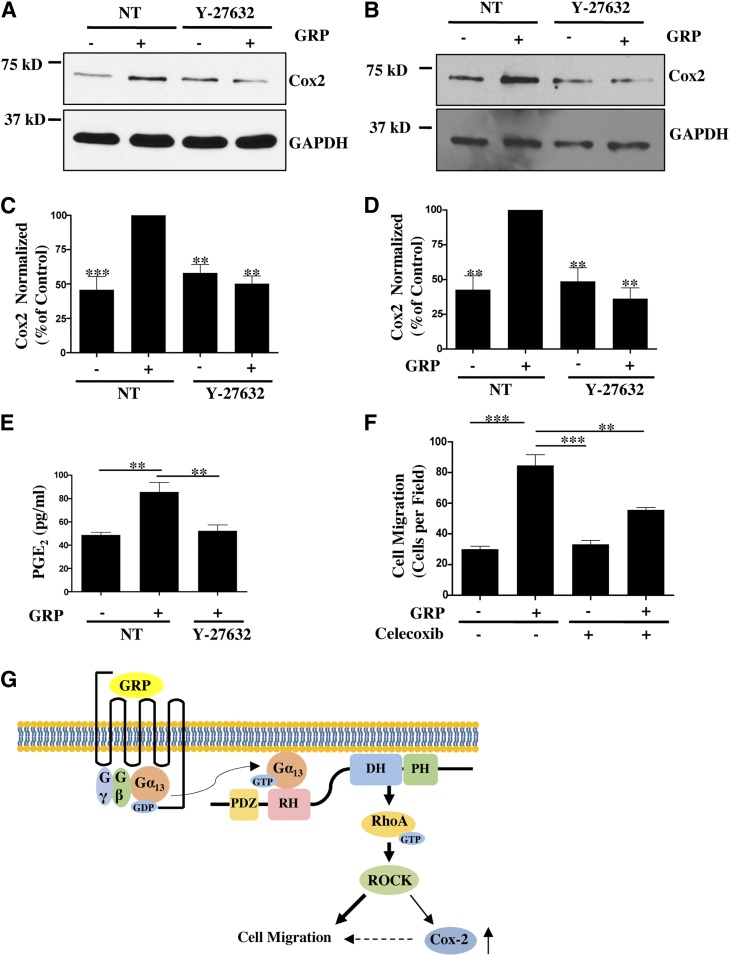
Having identified that PRG-RhoA signaling plays a role in GRP-stimulated Cox-2 expression, we questioned if this regulation is mediated though ROCK. ROCK has previously been implicated in regulation of Cox-2 expression in different tissues (Benitah et al., 2003; Hirota et al., 2012). Here we used Y-27632 (20 μM) to inhibit ROCK and assess its effect on Cox-2 expression in response to GRP. ROCK inhibition abrogates GRP-mediated stimulation of Cox-2 expression in Caco-2 and HT-29 cells (Fig. 8, A–D). We also observed that treatment with Y-27632 impedes GRP-stimulated PGE2 production (Fig. 8E). These data reveal that the PRG-RhoA-ROCK signaling axis downstream of GRPR activation contributes to Cox-2 expression and PGE2 production in colon cancer cells.
Fig. 8.
Rho-ROCK mediated regulation of Cox-2-PGE2production contributes to overall GRP stimulated cancer cell migration. (A,B) Cox-2 expression in Caco-2 (A) and HT-29 (B) cells treated with or without Y-27632 (20 μM) along with GRP for 8 hours. Cox-2 expression was verified by Western Blot. (C,D) Statistical densitometric analysis of Cox-2 expression in Caco-2 (C) and HT-29 (D) cells from n = 3. Shown are mean values ± S.E.M.; ** P < 0.01, ***P < 0.001. (E) Caco-2 cells serum-starved overnight and stimulated with GRP with or without Y-27632 for 8 hours. Cell media for each condition was harvested and analyzed for PGE2 concentration by enzyme-linked immunosorbent assay (ELISA) (see Materials and Methods). Statistical analysis of n = 3. Shown are mean values ± S.E.M.; **P < 0.01. (F) Caco-2 cells serum-starved overnight and plated on the upper chamber of Transwell insert at 5 × 105 cells/well. Transwell inserts were contained in media supplemented with 1% FBS and with or without GRP along with celecoxib (20 μM) (see Materials and Methods). Statistical analysis of cell migration, n = 3 repeated in duplicates. Shown are mean values ± S.E.M.; **P < 0.01, ***P < 0.001. (G) Model of GRPR signaling in colon cancer cells. GRP stimulation increases RhoA activity primarily through Gα13-PRG activation. PRG-RhoA-ROCK signaling axis is required for GRP-stimulated colon cancer cell migration (denoted by bolder arrows). PRG-RhoA-ROCK also regulates Cox-2 expression, which makes a small contribution (denoted by dotted arrow) to overall GRP-stimulated colon cancer cell migration (see text for details).
Evidence from in vitro and in vivo studies have shown that Cox-2-PGE2 signaling increases colon cancer cell migration and invasion (Buchanan et al., 2003; Hawcroft et al., 2012; Ninomiya et al., 2012). Therefore, we wanted to identify the contribution of Cox-2-PGE2 signaling to overall GRP-stimulated colon cancer cell migration. Here we conducted a Transwell cell migration assay with Caco-2 cells stimulated with GRP incubated with or without celecoxib, a Cox-2-specific inhibitor. It has been reported that celecoxib at 20 μM does not result in colon cancer cell apoptosis (Xiao et al., 2008). Caco-2 cells incubated with celecoxib without GRP had no defect in basal cell migration in comparison with dimethyl sulfoxide–treated Caco-2 cells (Fig. 8F). However, celecoxib treatment did result in a modest reduction (∼35%) in GRP-stimulated migration of Caco-2 cells as compared with Caco-2 cells treated with both GRP and dimethyl sulfoxide (Fig. 8F). Thus, our data indicates that Cox-2 expression and activity contributes to overall GRP-mediated colon cancer cell migration.
PRG Expression Is Upregulated in Colon Cancer Cells.
Our data show that PRG is critical in regulation of cell migration stimulated though GRPR. Enhanced propagation of GRPR signaling in colon cancer cells might be achieved by elevated expression of PRG. Evaluation of PRG protein levels demonstrated higher PRG expression in Caco-2 and HT-29 colon cancer cells compared with primary Human Colonic Epithelial Cells (HCoEpiC) and samples from normal human distal colonic mucosa (DCM) (Fig. 9A). Furthermore, analysis of copy number variation for RH-RhoGEFs in Catalogue of Somatic Mutations in Cancer database (COSMIC) revealed that 17.1% of the 486 tested human colon cancers have gains in PRG gene copy number (COSMIC v68) (Fig. 9B). These results indicate that PRG expression may be elevated in colon cancers, playing a critical role in regulation of colon cancer cell migration and invasion.
Fig. 9.
PRG expression is upregulated in colon cancer cells. (A) Protein expression of PRG in Caco-2, HT-29, primary human colonic epithelial cells, and two different samples of human distal colon mucosa. (B) Copy number variation (CNV) of three RH-RhoGEF family members in four common types of solid tumors obtained from COSMIC v68. Depicting RH-RhoGEF gene gain or loss within these solid tumors.
Discussion
GPCRs coupled to Gα12/13 have been implicated in cancer progression via increased cancer cell migration and invasion in small-cell lung cancer, breast cancer, prostate cancer, and colon cancer (Lappano and Maggiolini, 2011). In our current work, we have identified the molecular mechanism by which GRPR-activated Gα13 signaling contributes to colon cancer cell migration. We have found that Gα13 is the predominant mediator of RhoA activation downstream of GRPR, whereas Gαq makes small contributions to total RhoA activation. This observation, along with previous studies demonstrating that chemokine (C-X-C) motif receptor 4 (CXCR4) and lysophosphatidic acid (LPA) receptors mediate RhoA activation through Gα13 (Bian et al., 2006; Yagi et al., 2011), suggests that Gα13 possibly is the predominant regulator of RhoA activity downstream of multiple GPCRs that couple to Gα12/13.
Our studies identify PRG as the predominant RH-RhoGEF activated downstream of GRPR in colon cancer cells, whereas the other two RH-RhoGEFs, p115 and LARG, have little or no change in activity as indicated by our RhoAG17A pulldown data. Interestingly, downregulation of PRG expression leads to a similar decrease in RhoA activation as inhibition of Gα13, indicating that Gα13 regulates RhoA through PRG. The remaining Gαq-mediated contribution to activation of RhoA may possibly be regulated through Trio, Kalirin, LARG, or p63RhoGEF. A previous report has demonstrated that LARG may be a downstream effector of Gαq (Booden et al., 2002); however, our RhoAG17A pulldown data does not support this possibility, as GRP stimulation brings about no further increase in LARG activation in colon cancer cells. It has been shown that p63RhoGEF, a RhoA-specific GEF, is activated through the direct interaction of AlF4-activated Gαq subunit with the C-terminal extension of p63RhoGEF’s PH domain (Lutz et al., 2005; Rojas et al., 2007). Indeed, we have observed that p63RhoGEF is activated upon GRP stimulation in Caco-2 cells and thus presumably contributes to Gαq-mediated RhoA activation (Patel and Kozasa, unpublished data). However, this novel pathway requires further characterization.
GPCR-mediated RhoA-ROCK activation plays a critical role in cell migration. Here, we report for the first time that GRP-stimulated colon cancer cell migration is regulated by the PRG-RhoA-ROCK signaling axis. Our findings are in line with previous studies, which have reported that PRG-RhoA-ROCK signaling regulates fibroblast cell migration and breast cancer cell migration. These studies have identified that PRG-RhoA-ROCK signaling regulates cell migration through induction of adhesion complexes and spatial regulation of actinomyosin contractile machinery (Iwanicki et al., 2008; Struckhoff et al., 2013). Prior work has also demonstrated that growth factor receptor tyrosine kinases also use Rho-ROCK signaling to promote tumor cell migration and invasion (Somlyo et al., 2000; Harrison et al., 2013). Hence, it is clear that ROCK can be an ideal molecular target for prevention of tumor cell migration and metastasis.
GPCR-mediated regulation of Cox-2 expression contributes to colon cancer progression by regulating proliferation, migration, and invasion (Wang and Dubois, 2010). Here we show that GRP stimulation of Caco-2 and HT-29 cells leads to Cox-2 expression. Our data for the first time supports the role of PRG in regulation of Cox-2 expression and Cox-2-mediated PGE2 production. Furthermore, we identified that ROCK, acting downstream of PRG-RhoA, contributes to Cox-2 expression in response to GRP stimulation. Our findings are in line with other studies that have also reported the role of ROCK in regulating Cox-2 expression downstream of another GPCR, proteinase-activated receptor-2 (Hirota et al., 2012). Current evidence indicates that Cox-2-PGE2 signaling stimulates colon cancer cell migration through activation of its cognate prostaglandin E receptor 4 or through transactivation of epidermal growth factor receptor (Buchanan et al., 2003; Hawcroft et al., 2012). Overall our data suggests that the modest defect in cancer cell motility observed with celecoxib treatment indicates that Cox-2-PGE2 signaling is not the main regulator of GRP-stimulated colon cancer cell migration, and most probably it is predominantly controlled by PRG-RhoA-ROCK pathway directly regulating actinomyosin contractile machinery.
The role of RH-RhoGEFs in tumorigenesis has just recently gained recognition. Existing evidence suggests that the role of these RH-RhoGEFs is varied in tumor development and metastasis and their functions are tumor-specific. It has been reported that p115 expression is upregulated in prostate cancer cells and invasive prostate tumors (Huang et al., 2011). However, the role of p115 in the context of its involvement in signaling downstream of GPCRs and its effect on cancer progression is not known. In contrast to elevated expression of p115 in prostate cancer, LARG expression in breast and colon cancers is reported to be decreased. In these cancers, LARG has been reported to act as a tumor suppressor (Ong et al., 2009). Loss of LARG expression in breast and colon cancer is also supported by data that shows that there is loss of gene copy number of LARG in these solid tumors. Here, we show that PRG is the major mediator of GRP-stimulated colon cancer cell migration. Data form the COSMIC database shows that PRG gene copy number is increased in a significant number of colon cancer samples. Furthermore, our results demonstrate that PRG is overexpressed in colon cancer cell lines. These results suggest that PRG may play a key role in regulation of tumorigenesis mediated by GRPR and other GPCRs. Indeed, a recent report by Struckhoff et al. (2013) concludes that PRG is essential for CXCR4-mediated breast cancer cell migration and invasion and found that PRG expression is increased at the leading edge of primary tumors and tumor cells that have undergone lymphatic invasion. Another study looking at PC-3 prostate cancer cells grown in three-dimensional organotypic culture reported increased PRG expression in the invasive cultures (Harma et al., 2012). Furthermore, PRG has been implicated as a prosurvival gene in human glioblastoma, where knockdown of PRG resulted in decreased cell viability (Thaker et al., 2009; Masica and Karchin, 2011). Thus, it is tempting to hypothesize that PRG expression and activity might be increased not only in colon cancer but also in other solid tumors, regulating prosurvival pathways, cancer cell migration, and invasion.
Characterization of GRPR-mediated signaling pathway in colon cancer cells has revealed new potential therapeutic targets. Identification of the role of PRG in GRPR-mediated colon cancer cell migration and Cox-2 expression opens additional opportunities for developing novel therapeutic agents. Application of a recently developed inhibitor specific for RH-RhoGEFs (Y16) together with existing inhibitors for Cox-2 may prove to have therapeutic effects on colon cancer models (Shang et al., 2013). As the roles of the RH-RhoGEFs in tumorigenesis and metastasis become more well-defined, development of novel inhibitors specific for p115, LARG, or PRG would expand our choices for selection of therapeutic strategy.
Supplementary Material
Abbreviations
- Cox-2
cyclo-oxygenase isoform 2
- FBS
fetal bovine serum
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- GRP
gastrin-releasing peptide
- GRPR
GRP receptor
- GST
glutathione S-transferase
- HEK
human embryonic kidney
- LARG
leukemia-associated RhoGEF
- p115
p115RhoGEF
- PDZ
postsynaptic density 95/disk-large/ZO-1
- PRG
PDZ-RhoGEF
- PGE2
prostaglandin E2
- RGS
regulator of G-protein signaling
- RH
regulator of G-protein signaling homology domain
- ROCK
Rho-associated kinase
- siRNA
small-interfering RNA
- Y-27632
(1R,4r)-4-((R)-1-aminoethyl)-N-(pyridin-4-yl)cyclohexanecarboxamide
Authorship Contributions
Participated in research design: Patel, Kozasa, Karginov.
Conducted experiments: Patel, Kawano.
Contributed new reagents or analytic tools: Suzuki, Hamakubo.
Performed data analysis: Patel, Kozasa, Karginov.
Wrote or contributed to the writing of the manuscript: Patel, Kozasa, Karginov.
Footnotes
References
- Aittaleb M, Boguth CA, Tesmer JJG. (2010) Structure and function of heterotrimeric G protein-regulated Rho guanine nucleotide exchange factors. Mol Pharmacol 77:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah SA, Valerón PF, Lacal JC. (2003) ROCK and nuclear factor-kappaB-dependent activation of cyclooxygenase-2 by Rho GTPases: effects on tumor growth and therapeutic consequences. Mol Biol Cell 14:3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian D, Mahanivong C, Yu J, Frisch SM, Pan ZK, Ye RD, Huang S. (2006) The G12/13-RhoA signaling pathway contributes to efficient lysophosphatidic acid-stimulated cell migration. Oncogene 25:2234–2244. [DOI] [PubMed] [Google Scholar]
- Booden MA, Siderovski DP, Der CJ. (2002) Leukemia-associated Rho guanine nucleotide exchange factor promotes G α q-coupled activation of RhoA. Mol Cell Biol 22:4053–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan FG, Wang D, Bargiacchi F, DuBois RN. (2003) Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem 278:35451–35457. [DOI] [PubMed] [Google Scholar]
- Carman CV, Parent J-L, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T. (1999) Selective regulation of Galpha(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem 274:34483–34492. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Matkowskyj KA, Chakrabarti S, McDonald TJ, Benya RV. (1999) Aberrant expression of gastrin-releasing peptide and its receptor by well-differentiated colon cancers in humans. Am J Physiol 276:G655–G665. [DOI] [PubMed] [Google Scholar]
- Carroll RE, Ostrovskiy D, Lee S, Danilkovich A, Benya RV. (2000) Characterization of gastrin-releasing peptide and its receptor aberrantly expressed by human colon cancer cell lines. Mol Pharmacol 58:601–607. [DOI] [PubMed] [Google Scholar]
- Chen Z, Singer WD, Danesh SM, Sternweis PC, Sprang SR. (2008) Recognition of the activated states of Galpha13 by the rgRGS domain of PDZRhoGEF. Structure 16:1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral RS, Iñiguez MA, Duque J, López-Pérez R, Fresno M. (2007) Bombesin induces cyclooxygenase-2 expression through the activation of the nuclear factor of activated T cells and enhances cell migration in Caco-2 colon carcinoma cells. Oncogene 26:958–969. [DOI] [PubMed] [Google Scholar]
- Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. (1985) Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature 316:823–826. [DOI] [PubMed] [Google Scholar]
- Ferris HA, Carroll RE, Rasenick MM, Benya RV. (1997) Constitutive activation of the gastrin-releasing peptide receptor expressed by the nonmalignant human colon epithelial cell line NCM460. J Clin Invest 100:2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G, Just I, Kaina B. (1999) Rho GTPases are over-expressed in human tumors. Int J Cancer 81:682–687. [DOI] [PubMed] [Google Scholar]
- Frucht H, Gazdar AF, Park JA, Oie H, Jensen RT. (1992) Characterization of functional receptors for gastrointestinal hormones on human colon cancer cells. Cancer Res 52:1114–1122. [PubMed] [Google Scholar]
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. (1999) A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem 274:5868–5879. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Chikumi H, Gutkind JS. (2000) Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett 485:183–188. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Wennerberg K, Arthur WT, Noren NK, Ellerbroek SM, and Burridge K (2006) Analysis of activated GAPs and GEFs in cell lysates, in GTPases Regulating Membrane Dynamics (Balch WE, Channing JD, Hall H eds) pp 425–437, Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- Glover S, Nathaniel R, Shakir L, Perrault C, Anderson RK, Tran-Son-Tay R, Benya RV. (2005) Transient upregulation of GRP and its receptor critically regulate colon cancer cell motility during remodeling. Am J Physiol Gastrointest Liver Physiol 288:G1274–G1282. [DOI] [PubMed] [Google Scholar]
- Härmä V, Knuuttila M, Virtanen J, Mirtti T, Kohonen P, Kovanen P, Happonen A, Kaewphan S, Ahonen I, Kallioniemi O, et al. (2012) Lysophosphatidic acid and sphingosine-1-phosphate promote morphogenesis and block invasion of prostate cancer cells in three-dimensional organotypic models. Oncogene 31:2075–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Knifley T, Chen M, O’Connor KL. (2013) LPA, HGF, and EGF utilize distinct combinations of signaling pathways to promote migration and invasion of MDA-MB-231 breast carcinoma cells. BMC Cancer 13:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, Sharma S, elMasry N, Qiu R-G, McCabe P, Polakis P, Bollag G. (1996) Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J Biol Chem 271:25452–25458. [DOI] [PubMed] [Google Scholar]
- Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. (1998) Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280:2112–2114. [DOI] [PubMed] [Google Scholar]
- Hawcroft G, Volpato M, Marston G, Ingram N, Perry SL, Cockbain AJ, Race AD, Munarini A, Belluzzi A, Loadman PM, et al. (2012) The omega-3 polyunsaturated fatty acid eicosapentaenoic acid inhibits mouse MC-26 colorectal cancer cell liver metastasis via inhibition of PGE2-dependent cell motility. Br J Pharmacol 166:1724–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota CL, Moreau F, Iablokov V, Dicay M, Renaux B, Hollenberg MD, MacNaughton WK. (2012) Epidermal growth factor receptor transactivation is required for proteinase-activated receptor-2-induced COX-2 expression in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 303:G111–G119. [DOI] [PubMed] [Google Scholar]
- Hopkins AM, Pineda AA, Winfree LM, Brown GT, Laukoetter MG, Nusrat A. (2007) Organized migration of epithelial cells requires control of adhesion and protrusion through Rho kinase effectors. Am J Physiol Gastrointest Liver Physiol 292:G806–G817. [DOI] [PubMed] [Google Scholar]
- Huang C, Liu S, Miller RT. (2011) Role of p115RhoGEF in the regulation of extracellular Ca(2+)-induced choline kinase activation and prostate cancer cell proliferation. Int J Cancer 128:2833–2842. [DOI] [PubMed] [Google Scholar]
- Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, Parsons JT. (2008) FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J Cell Sci 121:895–905. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. (2008) International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson R, Pedersen ED, Wang Z, Brakebusch C. (2009) Rho GTPase function in tumorigenesis. Biochim Biophys Acta 1796:91–98. [DOI] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, Sternweis PM, Singer WD, Gilman AG, Bollag G, Sternweis PC. (1998) p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280:2109–2111. [DOI] [PubMed] [Google Scholar]
- Lappano R, Maggiolini M. (2011) G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov 10:47–60. [DOI] [PubMed] [Google Scholar]
- Lutz S, Freichel-Blomquist A, Yang Y, Rümenapp U, Jakobs KH, Schmidt M, Wieland T. (2005) The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem 280:11134–11139. [DOI] [PubMed] [Google Scholar]
- Makrodouli E, Oikonomou E, Koc M, Andera L, Sasazuki T, Shirasawa S, Pintzas A. (2011) BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study. Mol Cancer 10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masica DL, Karchin R. (2011) Correlation of somatic mutation and expression identifies genes important in human glioblastoma progression and survival. Cancer Res 71:4550–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikelis CM, Palmby TR, Simaan M, Li W, Szabo R, Lyons R, Martin D, Yagi H, Fukuhara S, Chikumi H, et al. (2013) PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem 288:12232–12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, Tani K, Kobayashi M, Maruyama T, Kobayashi K, et al. (2002) Involvement of prostaglandin E receptor subtype EP(4) in colon carcinogenesis. Cancer Res 62:28–32. [PubMed] [Google Scholar]
- Ninomiya I, Nagai N, Oyama K, Hayashi H, Tajima H, Kitagawa H, Fushida S, Fujimura T, Ohta T. (2012) Antitumor and anti-metastatic effects of cyclooxygenase-2 inhibition by celecoxib on human colorectal carcinoma xenografts in nude mouse rectum. Oncol Rep 28:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong DCT, Ho YM, Rudduck C, Chin K, Kuo WL, Lie DKH, Chua CLM, Tan PH, Eu KW, Seow-Choen F, et al. (2009) LARG at chromosome 11q23 has functional characteristics of a tumor suppressor in human breast and colorectal cancer. Oncogene 28:4189–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic S, Miller G, Schally AV. (1991) Inhibition of growth of HT-29 human colon cancer xenografts in nude mice by treatment with bombesin/gastrin releasing peptide antagonist (RC-3095). Cancer Res 51:6006–6009. [PubMed] [Google Scholar]
- Ren X-D and Schwartz MA (2000) Determination of GTP loading on Rho. Methods Enzymol 325:264–272. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399. [DOI] [PubMed] [Google Scholar]
- Rigas B, Goldman IS, Levine L. (1993) Altered eicosanoid levels in human colon cancer. J Lab Clin Med 122:518–523. [PubMed] [Google Scholar]
- Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J. (2007) Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain. J Biol Chem 282:29201–29210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. (1998) Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol 177:507–517. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. (2002) RHO-GTPases and cancer. Nat Rev Cancer 2:133–142. [DOI] [PubMed] [Google Scholar]
- Scheschonka A, Dessauer CW, Sinnarajah S, Chidiac P, Shi C-S, Kehrl JH. (2000) RGS3 is a GTPase-activating protein for g(ialpha) and g(qalpha) and a potent inhibitor of signaling by GTPase-deficient forms of g(qalpha) and g(11α). Mol Pharmacol 58:719–728. [DOI] [PubMed] [Google Scholar]
- Shang X, Marchioni F, Evelyn CR, Sipes N, Zhou X, Seibel W, Wortman M, Zheng Y. (2013) Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors. Proc Natl Acad Sci USA 110:3155–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer WD, Miller RT, Sternweis PC. (1994) Purification and characterization of the alpha subunit of G13. J Biol Chem 269:19796–19802. [PubMed] [Google Scholar]
- Slice LW, Walsh JH, Rozengurt E. (1999) Galpha(13) stimulates Rho-dependent activation of the cyclooxygenase-2 promoter. J Biol Chem 274:27562–27566. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Bradshaw D, Ramos S, Murphy C, Myers CE, Somlyo AP. (2000) Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun 269:652–659. [DOI] [PubMed] [Google Scholar]
- Struckhoff AP, Rana MK, Kher SS, Burow ME, Hagan JL, Del Valle L, Worthylake RA. (2013) PDZ-RhoGEF is essential for CXCR4-driven breast tumor cell motility through spatial regulation of RhoA. J Cell Sci 126:4514–4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Kreutz B, Suzuki N, Kozasa T. (2004) Regulation of RGS-RhoGEFs by Gα12 and Gα13 Proteins. Methods Enzymol 390:285–294. [DOI] [PubMed] [Google Scholar]
- Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJG. (2005) Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science 310:1686–1690. [DOI] [PubMed] [Google Scholar]
- Thaker NG, Zhang F, McDonald PR, Shun TY, Lewen MD, Pollack IF, Lazo JS. (2009) Identification of survival genes in human glioblastoma cells by small interfering RNA screening. Mol Pharmacol 76:1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil D, Kim TY, Plachco A, Garton AJ, Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL, et al. (2012) ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res 72:5338–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnubhotla R, Sun S, Huq J, Bulic M, Ramesh A, Guzman G, Cho M, Glover SC. (2007) ROCK-II mediates colon cancer invasion via regulation of MMP-2 and MMP-13 at the site of invadopodia as revealed by multiphoton imaging. Lab Invest 87:1149–1158. [DOI] [PubMed] [Google Scholar]
- Wang D, Dubois RN. (2010) The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu M, Kozasa T, Rothstein JD, Sternweis PC, Neubig RR. (2004) Thrombin and lysophosphatidic acid receptors utilize distinct rhoGEFs in prostate cancer cells. J Biol Chem 279:28831–28834. [DOI] [PubMed] [Google Scholar]
- Wiznerowicz M, Trono D. (2003) Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol 77:8957–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Zhang Q, Lin Y, Reddy BS, Yang CS. (2008) Combination of atorvastatin and celecoxib synergistically induces cell cycle arrest and apoptosis in colon cancer cells. Int J Cancer 122:2115–2124. [DOI] [PubMed] [Google Scholar]
- Yagi H, Tan W, Dillenburg-Pilla P, Armando S, Amornphimoltham P, Simaan M, Weigert R, Molinolo AA, Bouvier M, Gutkind JS. (2011) A synthetic biology approach reveals a CXCR4-G13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal 4:ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang S, Zhang Z, He J, Xu Y, and Liu S (2013) ROCK has a crucial role in regulating prostate tumor growth through interaction with c-Myc. Oncogene DOI: 10.1038/onc.2013.505. [published ahead of print] [DOI] [PubMed]
- Zheng R, Iwase A, Shen R, Goodman OB, Jr, Sugimoto N, Takuwa Y, Lerner DJ, Nanus DM. (2006) Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene 25:5942–5952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.