Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor recognized for its role in xenobiotic metabolism. The physiologic function of AHR has expanded to include roles in immune regulation, organogenesis, mucosal barrier function, and the cell cycle. These functions are likely dependent upon ligand-mediated activation of the receptor. High-affinity ligands of AHR have been classically defined as xenobiotics, such as polychlorinated biphenyls and dioxins. Identification of endogenous AHR ligands is key to understanding the physiologic functions of this enigmatic receptor. Metabolic pathways targeting the amino acid tryptophan and indole can lead to a myriad of metabolites, some of which are AHR ligands. Many of these ligands exhibit species selective preferential binding to AHR. The discovery of specific tryptophan metabolites as AHR ligands may provide insight concerning where AHR is activated in an organism, such as at the site of inflammation and within the intestinal tract.
Introduction
The aryl hydrocarbon receptor (AHR) is a ligand activated transcription factor of the basic region helix-loop-helix-PER/ARNT/SIM homology family (Bersten et al., 2013). AHR was first identified in the early 1970s as the mediator of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD/dioxin) toxicity (Poland and Knutson, 1982). Overt dioxin exposure in humans leads to a chronic inflammatory condition known as chloracne, which is characterized by persistent painful skin lesions. The receptor contains a promiscuous ligand-binding pocket that can bind multiple classes of exogenous compounds such as polycyclic aromatic hydrocarbons and polychlorinated biphenyls (Poland and Knutson, 1982).
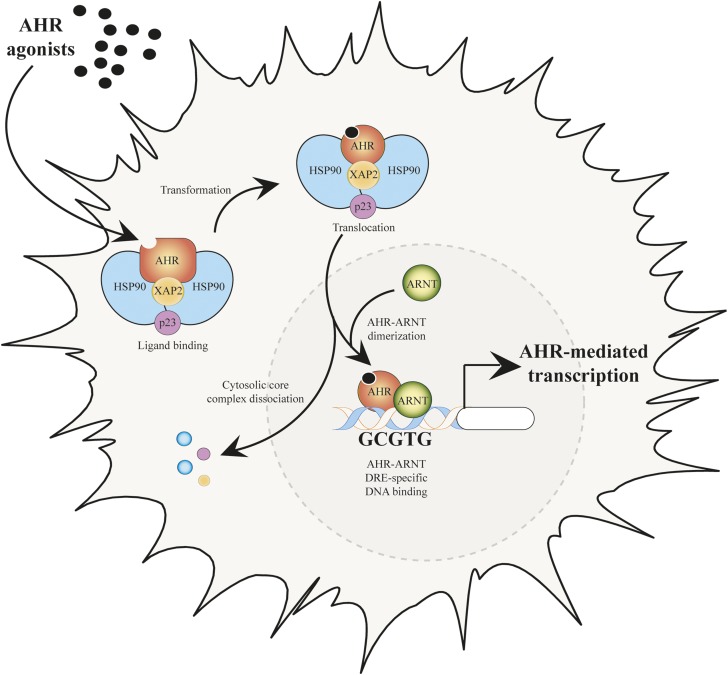
The prototypic signaling pathway of AHR-mediated transcriptional activity is characterized by transcription of a battery of drug-metabolizing enzymes, which includes cytochrome P450 enzymes 1A1, 1A2, and 1B1 (Denison et al., 1988; Strom et al., 1992; Rowlands and Gustafsson, 1997; Zhang et al., 1998; Nukaya et al., 2009). In the absence of ligand, AHR resides within the cytoplasm in a stable complex with two molecules of heat shock protein 90, one molecule of X-associated protein 2, and one molecule of the heat shock protein 90 cochaperone p23 (Fig. 1) (Perdew, 1988; Meyer et al., 1998; Kazlauskas et al., 1999). The AHR undergoes nucleocytoplasmic shuttling in the absence of ligand, but the significance of this ability remains to be established (Ikuta et al., 2000).
Fig. 1.
Canonical pathway of AHR-mediated transcription after binding.
AHR ligands enter the ligand-binding domain of the receptor, facilitating conformational changes that expose the nuclear localization sequence, which mediates active transport into the nucleus (Ikuta et al., 1998). Once localized to the nucleus, AHR dissociates from its cytoplasmic complex in the presence of the aryl hydrocarbon receptor nuclear translocator (ARNT), thus generating a functional heterodimeric transcription factor complex (Reyes et al., 1992). Formation of the AHR/ARNT heterodimer facilities binding to a consensus DNA sequence (5ʹ-TNGCGTG-3ʹ), referred to as a dioxin response element (DRE) (Shen and Whitlock, 1992). AHR/ARNT binding to a DRE leads to coactivator recruitment to the transactivation domain of AHR, which facilitates chromatin remodeling and subsequent target gene transcription (Fig. 1).
Most of the genes identified as direct targets of AHR activation were identified through exposure to potent AHR ligands, either in cell culture or in vivo without the presence of other stimuli. However, more recently it has been recognized that AHR can regulate gene transcription in the presence of other mediators, such as those involved in inflammatory signaling. Genes regulated during inflammation, such as IL6, IL22, PTGS2, and in angiogenesis, such as VEGFA, are known to be regulated by activation of AHR in a combinatorial fashion in the presence of inflammatory mediators (DiNatale et al., 2010b; Monteleone et al., 2011; Lahoti et al., 2013; Terashima et al., 2013). The diverse list of genes modulated by receptor activity suggests broad physiologic implications for AHR, beyond that of xenobiotic metabolism.
The Physiologic Significance of AHR
The physiologic effects of AHR activation have expanded to include multifaceted roles in immune regulation, intestinal homeostasis, and carcinogenesis. The AHR integrates into both innate and adaptive immunity through diverse mechanisms; including repression of acute phase response genes, differentiation of regulatory T cell (Treg) lymphocytes and B-cell differentiation (Funatake et al., 2005; Funatake et al., 2008; Patel et al., 2009; Bhattacharya et al., 2010; Mohinta et al., 2015). The AHR also promotes intestinal homeostasis through regulation and development of intraepithelial lymphocytes and innate lymphoid cells (Kiss et al., 2011; Li et al., 2011; Qiu et al., 2012). These cell types maintain a critical line of defense against infiltrating pathogenic microbes and facilitate gut homeostasis at the basolateral surface of the intestinal epithelium.
The involvement of AHR in inflammatory signaling and cell cycle progression suggests it may play a role in various stages of tumorigenesis (Gasiewicz et al., 2008; Safe et al., 2013; Murray et al., 2014). Evidence for this includes the high levels of constitutive receptor activity and nuclear localization observed in aggressive tumors and tumor cell lines (Yang et al., 2008; DiNatale et al., 2012; Richmond et al., 2014).
The measurement of AHR activity in the tumor microenvironment has been investigated as a possible diagnostic indicator of cancer aggressiveness (Murray et al., 2014). The results indicated a positive or negative correlation between AHR activity and a poor prognosis, which depends on the type of cancer. For example, studies have suggested that increased AHR activity is associated with diminished aggressiveness of hormone-dependent breast tumors, most likely due to AHR transrepression of estrogen receptor signaling (Saito et al., 2014). Conversely, elevated AHR transcriptional activity is observed with non-small-cell lung cancer and correlates with increased malignancy and poor prognosis (Su et al., 2013). These findings indicate the importance of ligand-mediated AHR signaling in vertebrates and suggest the existence of endogenous ligands that modulate inducible receptor activity.
The Case for Endogenous AHR Ligands
The expression of genes that exhibit a degree of AHR dependency is observed in the absence of exogenous xenobiotic exposure, suggesting either the existence of endogenously derived AHR ligands or additional mechanisms of regulation. For example, AHR/ARNT heterodimer has been observed to reside constitutively in the nucleus of HeLa cells (Singh et al., 1996). The advent of targeted gene disruption in mammalian systems has enabled the creation of the Ahr−/− mouse model to examine the in vivo physiologic functions of AHR in the absence of xenobiotic exposure (Schmidt et al., 1996). AHR null mice were found to be resistant to dioxin toxicity and failed to significantly express AHR target genes such as Cyp1a1 and Cyp1a2 (Fernandez-Salguero et al., 1995; Mimura et al., 1997). However, the absence of AHR expression during development led to a multitude of unexpected phenotypic abnormalities (Schmidt et al., 1996; Fernandez-Salguero et al., 1997). For example, the neonatal closure of the portal ductus venosus was found to depend on the presence of AHR in developing neonatal mice (Lahvis et al., 2005). Furthermore, altered organ development, including decreased liver size, impaired hepatic vascular development, cardiac hypertrophy, gastric lesions, and dermal fibrosis were observed in aged Ahr−/− mice. In addition, these mice are more susceptible to intestinal challenge, supporting a critical role for AHR in gut immune function and barrier function (Sutter et al., 2011). The decreased fitness of AHR null mice suggests extensive physiologic roles for AHR in developing mammals and supports the existence of endogenous ligands to invoke constitutive receptor activity.
Search for Endogenous Ligands
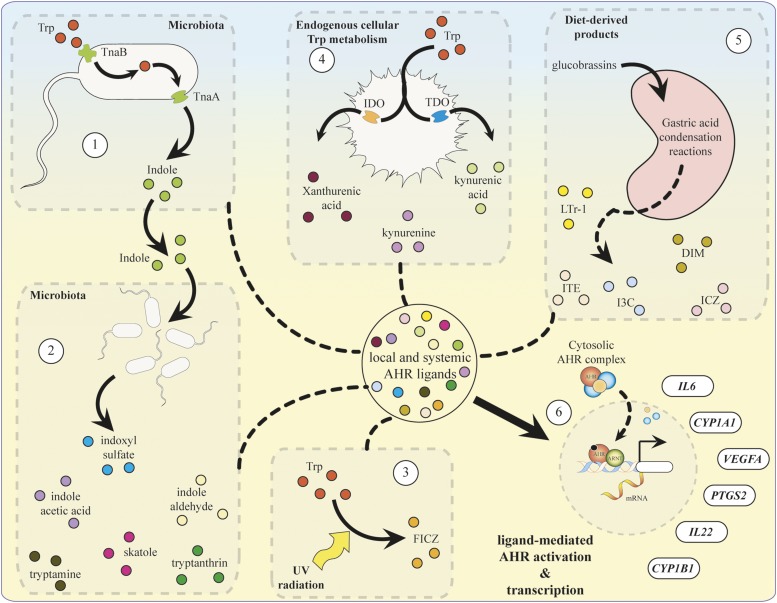
The search for putative endogenous AHR ligands and their physiologic role has been an expanding area of interest in the field of AHR biology (Denison and Nagy, 2003; Nguyen and Bradfield, 2008). The generation of endogenous ligands and subsequent AHR activation may occur by diverse mechanisms, such as dietary consumption, synthesis by host commensal microorganisms, free radical formation, and endogenous enzymatic activity. Interestingly, lack of AHR leads to accumulation of AHR ligands in the lung of AHR null mice (Chiaro et al., 2007).
Numerous compounds have been proposed as putative endogenous AHR ligands, many of which are generated through pathways involved in the metabolism of the amino acid tryptophan and indole (Bittinger et al., 2003; Chung and Gadupudi, 2011) (Table 1). In studies attempting to assess the agonist potential of a compound, it is important to test whether physiologically relevant concentrations can mediate AHR activity. The identification of endogenous metabolites that mediate induction of AHR target genes and nuclear translocation of the receptor should not be used as a proxy for ligand-binding assays to be able to definitively identify them as actual AHR ligands. A compound must be shown to occupy the ligand-binding domain of AHR to be characterized as an AHR ligand. To be defined as an AHR ligand, a given compound should be confirmed through either a binding assessment using a radioactive analog of the compound in question or a competitive ligand binding assay using a known radioactive AHR ligand (Ramadoss and Perdew, 2004). Identification of novel routes of endogenous ligand synthesis is critical to understanding the mechanisms of inducible receptor activation and how such activation promotes various states of health and disease.
TABLE 1.
List of endogenous AHR activators derived from indole or tryptophan metabolism
| Origin | Compound | Structure | MW | Confirmed Ligand | Human AHR Selective Agonist | Mouse AHR Selective Agonist | References |
|---|---|---|---|---|---|---|---|
| Exogenous | 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | 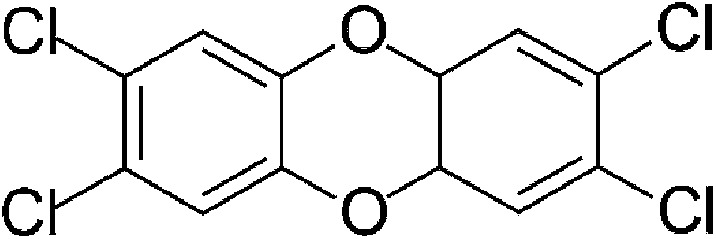 |
321.97 | ✓ | ✓ | Poland and Knutson, 1982 | |
| Dietary | lndole-3-carbinol (I3C) | 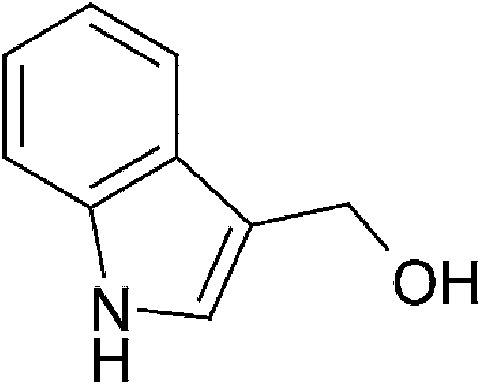 |
147.18 | ✓ | Bjeldanes et al., 1991; Ito et al., 2007 | ||
| Dietary | lndole-3-acetonitrile (I3ACN) | 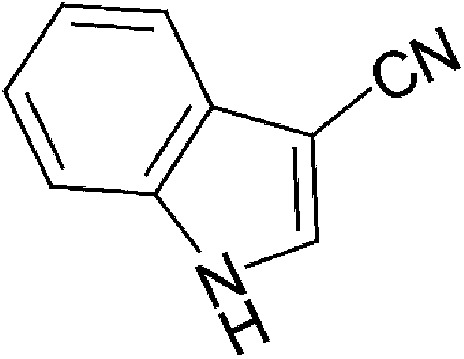 |
156.18 | ✓ | Bjeldanes et al., 1991 | ||
| Dietary | 3,3′-Diindolylmethane (DIM) | 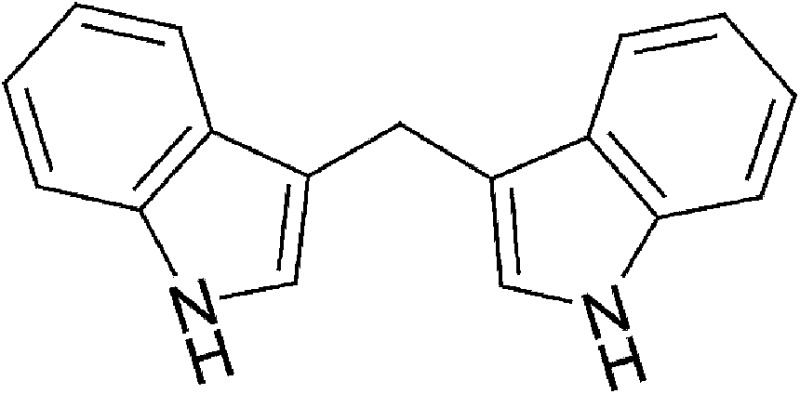 |
246.31 | ✓ | Bjeldanes et al., 1991 | ||
| Dietary | 2-(Indol-3-ylmethyl)-3,3′-diindolylmethane (Ltr-1) | 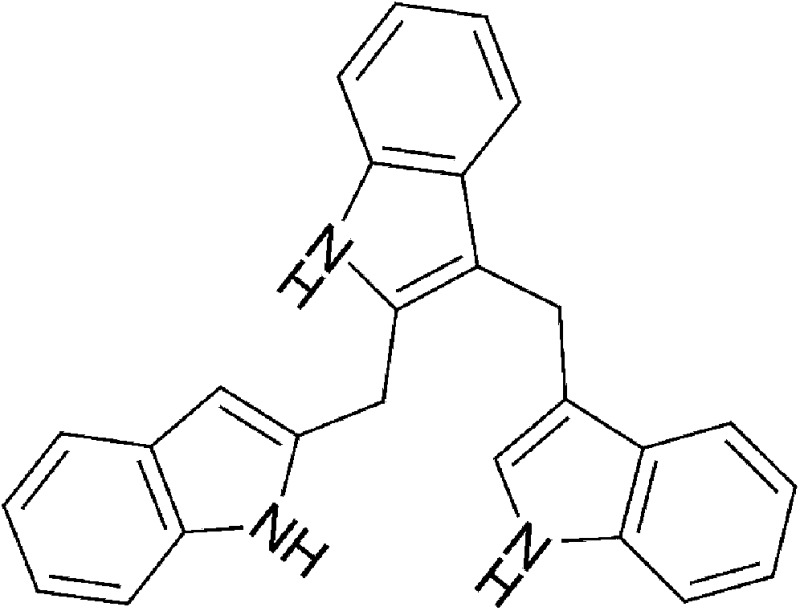 |
369.47 | ✓ | Bjeldanes et al., 1991 | ||
| Dietary | Indolo[3,2-b]carbazole (ICZ) | 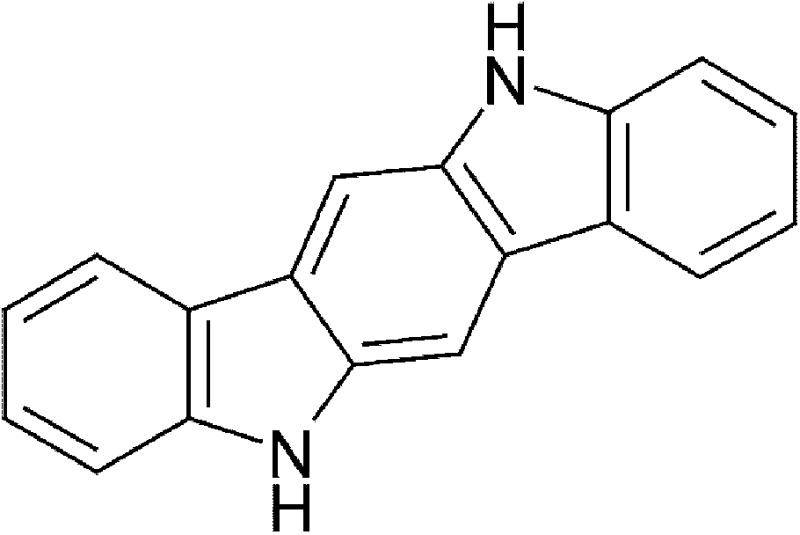 |
254.28 | ✓ | Bjeldanes et al., 1991; Jellinck et al., 1993 | ||
| Dietary | 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) | 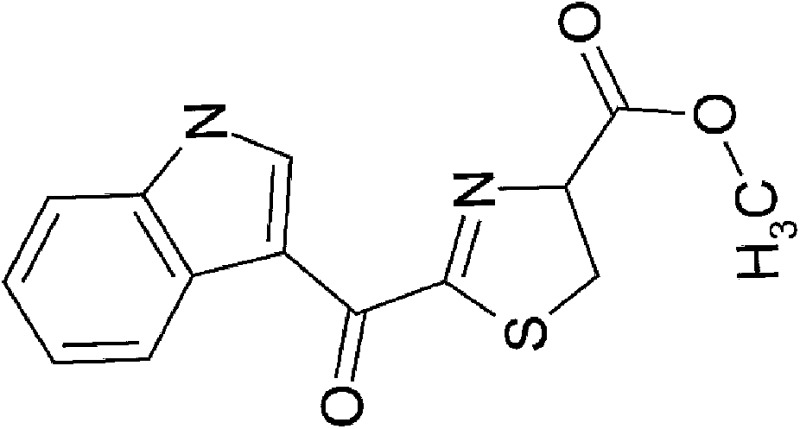 |
286.31 | ✓ | Song et al., 2002 | ||
| Microbial | Indole | 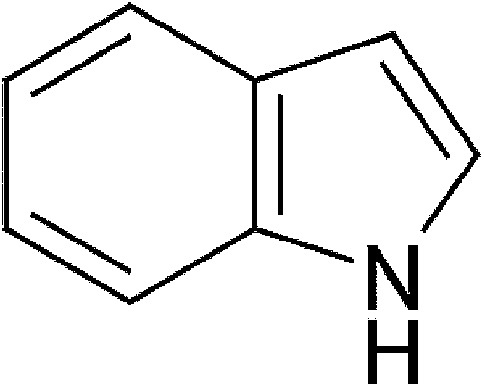 |
117.15 | ✓ | ✓ | Miller, 1997; Hubbard, 2015 | |
| Microbial | lndole-3-acetic acid (IAA) | 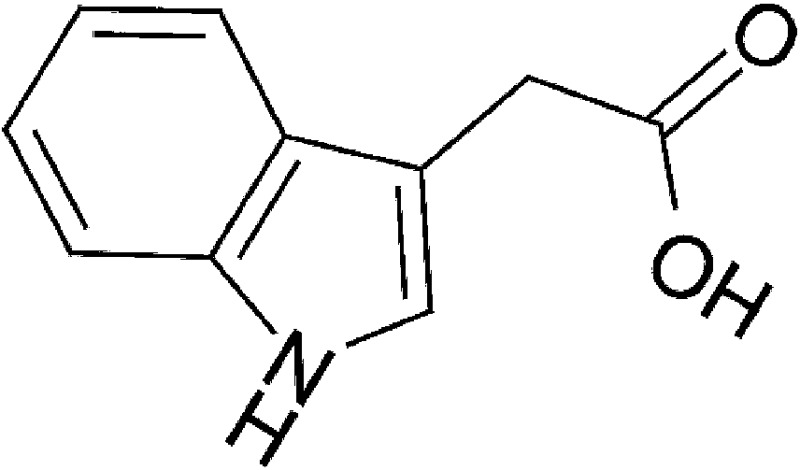 |
175.18 | ✓ | Miller, 1997; Jin et al., 2014 | ||
| Microbial | lndole-3-aldehyde (IAld) | 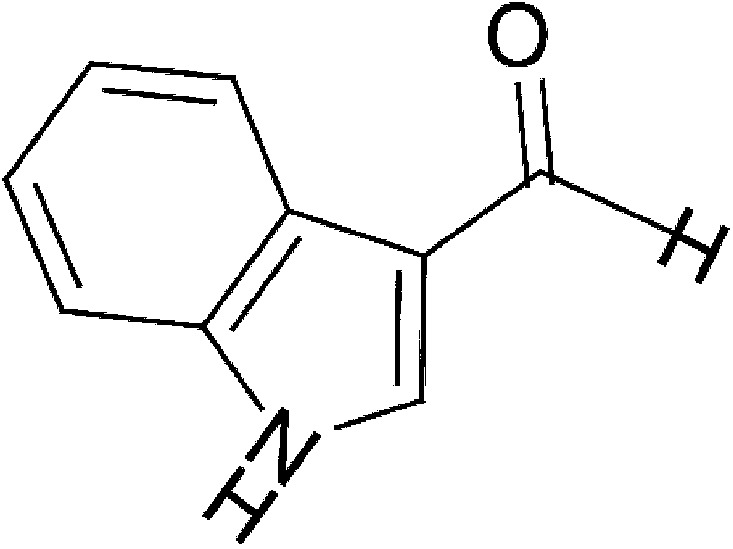 |
145.16 | ✓ | Bjeldanes et al., 1991; Zelante et al., 2013 | ||
| Microbial | Tryptamine | 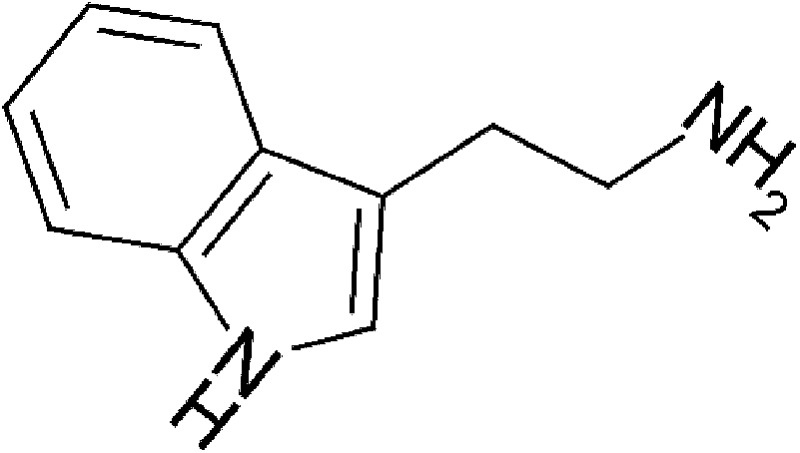 |
160.22 | ✓ | Miller, 1997; Jin et al., 2014 | ||
| Microbial | 3-Methyl-indole (skatole) | 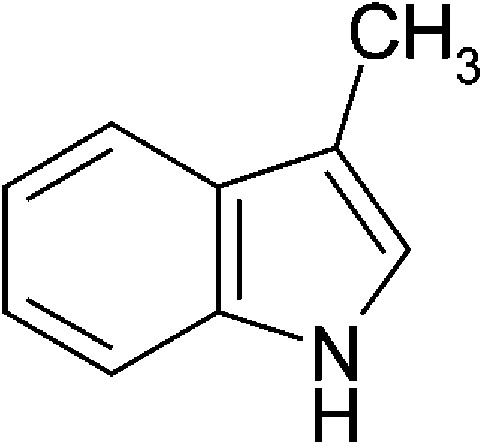 |
131.17 | * | Weems and Yost, 2010; Hubbard, 2015 | ||
| Yeast | Tryptamine | 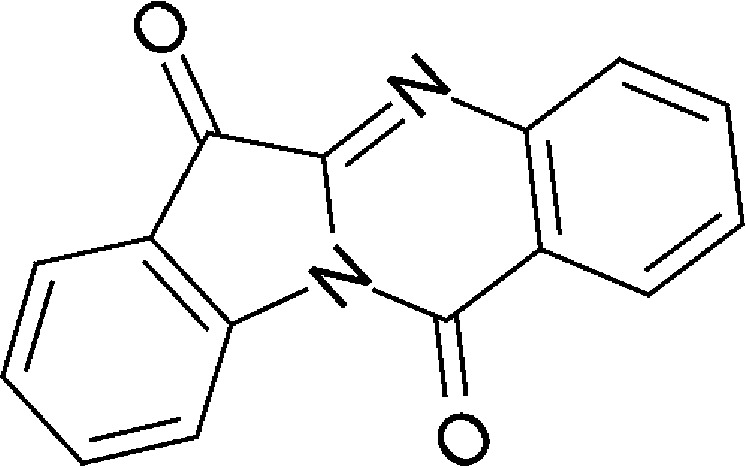 |
248.24 | * | Schrenk et al., 1999; Vlachos et al., 2012 | ||
| Microbial/host metabolism | Indigo | 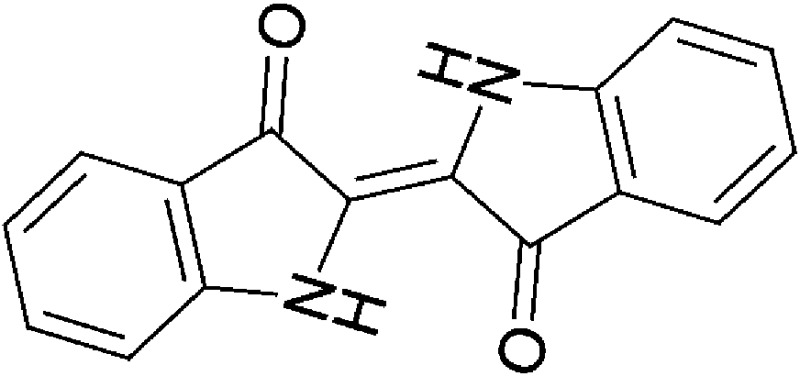 |
248.24 | ✓ | ✓ | Adachi et al., 2001; Peter Guengerich et al., 2004; Sugihara et al., 2004 | |
| Microbial/host metabolism | Indirubin |  |
262.27 | ✓ | ✓ | Adachi et al., 2001; Peter Guengerich et al., 2004; Sugihara et al., 2004; Flaveny et al., 2009 | |
| Microbial/host metabolism | lndoxyl-3-sulfate (I3S) | 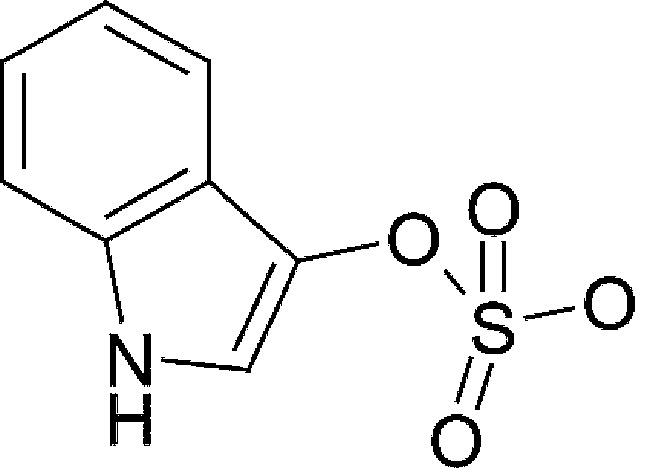 |
213.21 | ✓ | ✓ | Schroeder et al., 2010 | |
| Host metabolism | Kynurenine (Kyn) | 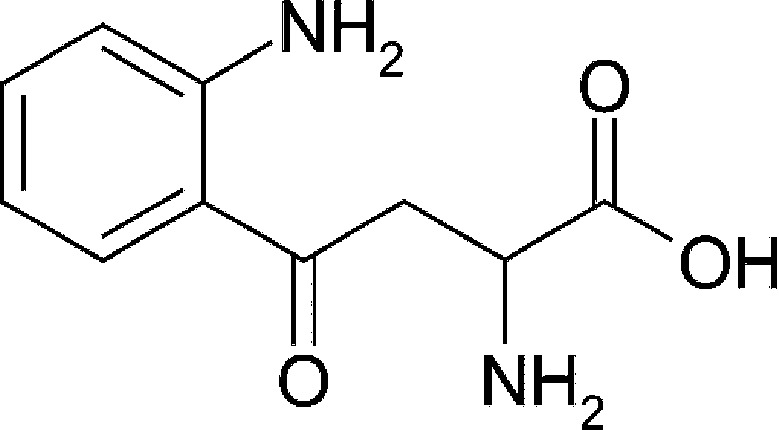 |
208.21 | ✓ | ✓ | Mezrich et al., 2010; Opitz et al., 2011 | |
| Host metabolism | Kynurenic acid (KA) | 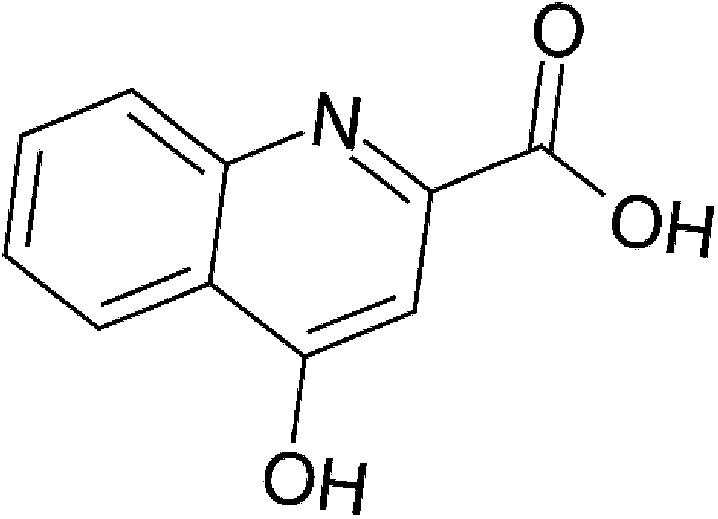 |
189.17 | ✓ | ✓ | DiNatale et al., 2010a | |
| Host metabolism | Xanthurenic acid | 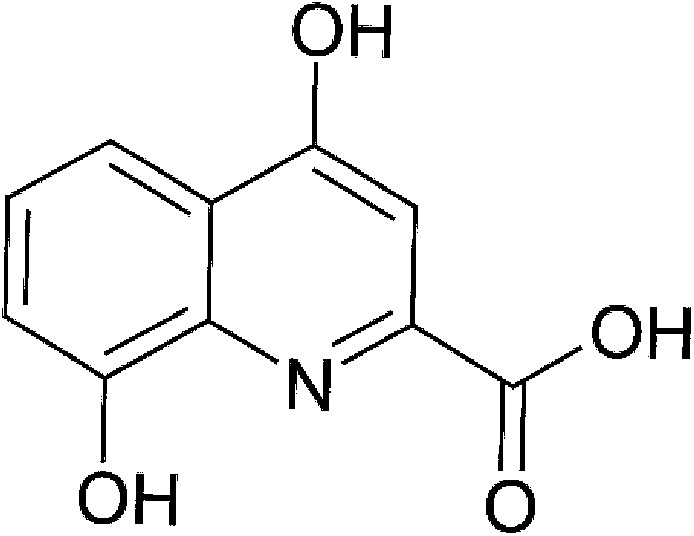 |
205.17 | * | ✓ | DiNatale et al., 2010a | |
| Host metabolism | Cinnabarinic acid (CA) | 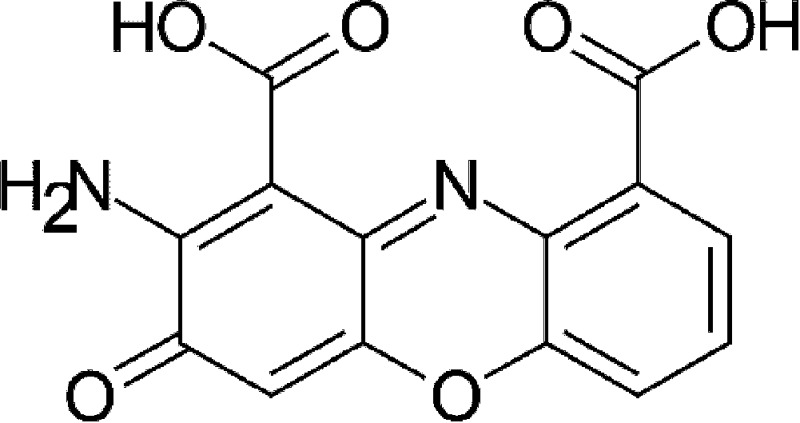 |
300.22 | * | Lowe et al., 2014 | ||
| UV-light oxidation | 6-Formylindolo[3,2-b]carbazole (FICZ) | 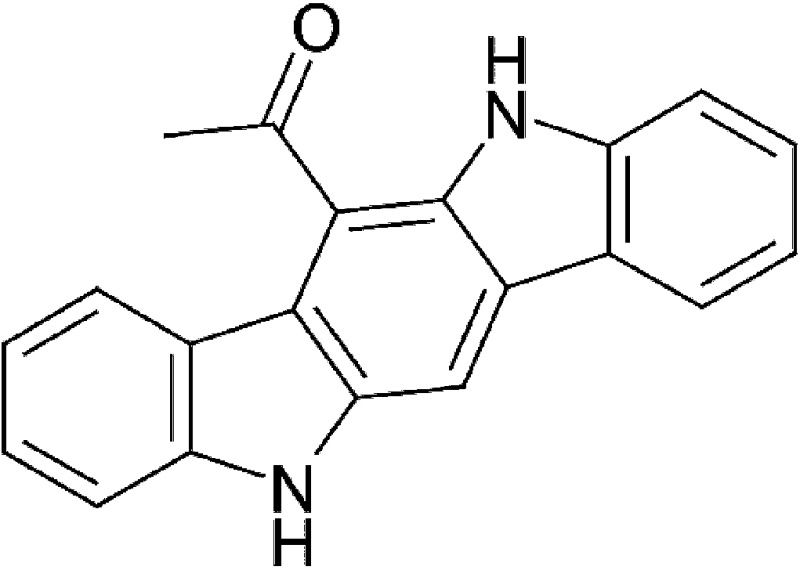 |
284.31 | ✓ | Rannug et al., 1987, 1995 |
MW, molecular weight.
Molecule is an activator of AHR, but further ligand binding assays are required.
Dietary Exposure to Indole-3-Carbinol
The absence of a primary endogenous regulator of AHR activation suggests multiple routes of ligand accumulation within an organism, including dietary intake. Consumption of AHR ligands could play a significant role in AHR-mediated effects on intestinal homeostasis and the local metabolism of dietary and endogenous compounds. The physiologic significance of AHR signaling in the gastrointestinal tract has been demonstrated using dextran sodium sulfate (DSS)-inducible intestinal injury models in which AHR null mice exhibit increasingly severe symptoms and lethality (Arsenescu et al., 2011; Benson and Shepherd, 2011). Activation of AHR by TCDD in intestinal disease models mediated increased survivability and decreased severity of symptoms (Takamura et al., 2010). However, colitis symptoms were significantly reduced in AHR heterozygous compared with AHR homozygous mice, suggesting that overstimulation of AHR may have mixed consequences (Arsenescu et al., 2011). Dietary intake of AHR agonists would likely provide a diverse array of potential ligands of varying potency and metabolic half-lives that could mediate systemic or localized receptor activation.
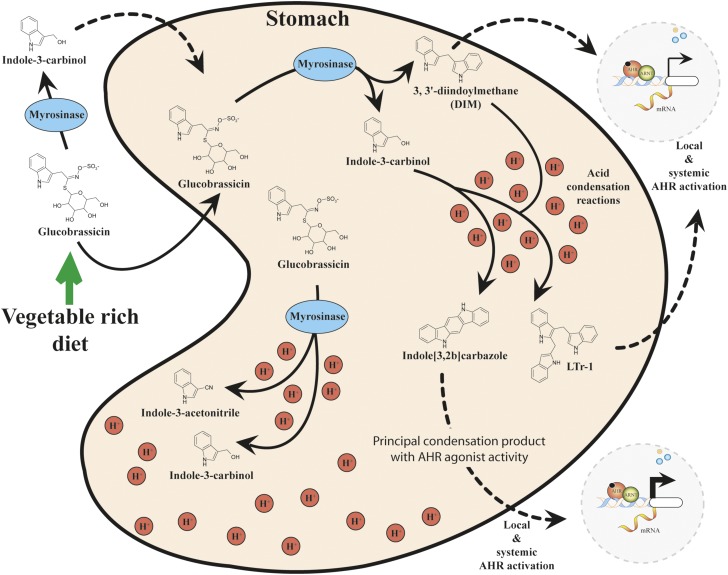
Biological analysis of extracts from herbal supplements, fruits, and vegetables has revealed significant activation of DRE-driven cell-based reporter systems, target gene expression, and DNA-binding functions, indicative of the presence of AHR activators (Jeuken et al., 2003). Cruciferous vegetables such as broccoli and brussels sprouts have been found to be rich sources of potent indole-based glucobrassicin (Fig. 2). Mastication promotes the enzymatic cleavage of glucobrassicin by myrosinases to yield indole-3-carbinol (I3C) and indole-3-acetonitrile (I3ACN), both of which exhibit the capacity to bind and activate AHR, albeit weakly (Loub et al., 1975; Bjeldanes et al., 1991; Shapiro et al., 2001; Ito et al., 2007). The presence of myrosinase activity within plant cells and resident microflora may contribute to the enzymatic cleavage of glucobrassicin to I3C (Dosz et al., 2014; Luang-In et al., 2014).
Fig. 2.
Gastric acid condensation of indole-3-carbinol lead to the production of high-affinity AHR ligands.
Interestingly, the in vivo potency of I3C and I3ACN with regard to AHR activation has been demonstrated to depend on the mode of exposure. Administration through intraperitoneal injection renders these compounds less potent compared with oral gavage (Bradfield and Bjeldanes, 1987). Subsequent studies revealed that in the presence of gastric acid both I3C and I3ACN undergo nonenzymatic acid condensation reactions to generate a number of additional AHR ligands, including 3,3′diindolylmethane, 2-(indol-3-ylmethyl)-3,3′-diindolylmethane, and indolo[3,2-b]carbazole (ICZ) (Bjeldanes et al., 1991). Of these glucobrassicin-derived compounds (I3C, I3ACN, 3,3′diindolylmethane, 2-(indol-3-ylmethyl)-3,3′-diindolylmethane, and ICZ), ICZ exhibits the highest affinity and activation potential for AHR and thus probably represents a physiologically relevant glucobrassicin-derived AHR ligand. Through the use of a competitive binding assay, ICZ was found to have a Kd of 190 pM, 27-fold less potent than TCDD (Bjeldanes et al., 1991; Jellinck et al., 1993).
The beneficial effects of I3C and its condensation products are wide ranging. Effects of I3C treatment upon suppression and initiation of carcinogenesis vary and appear to be dependent upon the experimental models used. Inhibition of tumorigenesis by I3C has been observed in various target tissues, including liver, thyroid, skin, lung, and colon (Fares, 2014). Anticarcinogenic activity within animal models of mammary and uterine tumorigenesis suggest I3C may have therapeutic potential in the treatment of human breast cancer (Bradlow et al., 1991; Kojima et al., 1994; Reed et al., 2005). This therapeutic function may be due to its antiestrogenic activity and inhibition of cell proliferation by estrogen signaling (Michnovicz and Bradlow, 1990; Marconett et al., 2010). Dietary I3C is reported to suppress in vivo models of prostate carcinogenesis by inducing cell cycle arrest and apoptosis of prostate cancer cells (Chinni et al., 2001; Nachshon-Kedmi et al., 2003; Sarkar and Li, 2004).
Dietary I3C or its condensation products have displayed therapeutic activity in mouse models of multiple sclerosis and DSS-induced colitis (Huang et al., 2013; Rouse et al., 2013). Both murine disease models are characterized by the induction of harmful inflammatory states that were alleviated through the AHR-dependent promotion of anti-inflammatory Treg differentiation. The dietary metabolite I3C has been shown to limit proinflammatory responses through additional mechanisms such as suppression of nuclear factor κB signaling pathways (Safa et al., 2015). Additionally, I3C or its metabolites exhibit antimicrobial properties, such as inhibition of Escherichia coli and Staphylococcus aureus biofilm formation (Monte et al., 2014). These effects may have significant implications upon host intestinal microbiota composition and may be a deterrent to opportunistic infection.
Despite the beneficial effects of I3C, its metabolites, or condensation products, long-term in vivo treatment studies have indicated that, consistent with its activity as an AHR agonist, I3C may be procarcinogenic and aid in the initiation of tumorigenesis. I3C administration within animal models has been shown to enhance the development of colonic lesions in rats and to promote aflatoxin B-1–initiated hepatocarcinogenesis in trout models (Oganesian et al., 1999; Exon et al., 2001). Recent investigations by the National Toxicology Program division of the National Institutes of Health have indicated that I3C administration to rodents correlates with an increase in hepatocarcinogenesis (NTP, 2014). This effect may be due to systemic overstimulation of AHR facilitated by excessive amounts of ligand.
Currently, I3C is available as a dietary supplement at dosages exceeding levels achievable with normal consumption of vegetables. Dietary levels of I3C most likely would facilitate beneficial localized activation of the AHR in the intestinal tract, while avoiding the potentially detrimental effects of systemic activation, further supporting the notion that less is more.
Microbiota-Derived AHR Ligands
The gastrointestinal tract is composed of a complex microbial ecosystem that contributes significantly to the first-pass metabolism of dietary constituents. The coevolutionary commensalism between host and microbes may include the catabolism of tryptophan to AHR ligands. Such interkingdom signaling pathways may have evolved to promote the maintenance of intestinal health through the sensing of bacteria at the epithelial interface. In contrast, the excessive production of AHR ligands could play a role in the etiology of various intestinal disease states. Microflora-mediated activation of AHR has been observed in murine models after oral gavage of heat-killed Lactobacillus bulgaricus OLL1811, which was found to attenuate DSS-induced colitis (Takamura et al., 2011). The metabolite responsible for the observed therapeutic activity remains to be identified. This concept is of further interest in the field of prebiotics and probiotics, where the mechanisms facilitating their beneficial effects remain to be fully elucidated.
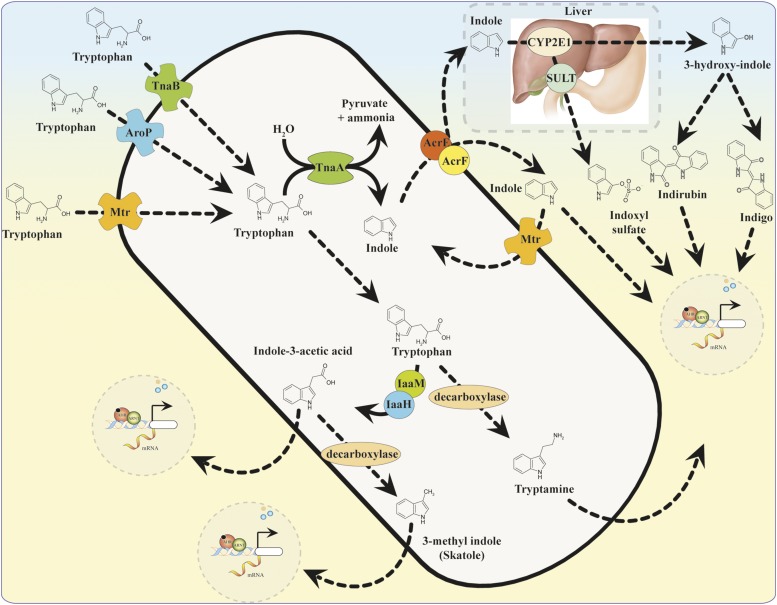
Microbial metabolism of the amino acid tryptophan is recognized as the source for the generation of a number of AHR ligands (Fig. 3). Tryptophan is an essential amino acid that can only be acquired through the diet due to its inability to be synthesized de novo. Resident microbes such as E. coli may use tryptophan as a source of nitrogen and contain up to three distinct permeases responsible for its transport: Mtr, AroP, and TnaB (Yanofsky et al., 1991). Tryptophan can be converted to mono-substituted indole compounds, such as indole-3-acetic acid (IAA) and tryptamine, that have been found to activate AHR in a yeast DRE-dependent reporter assay and stimulate target gene expression in a human colon-carcinoma (Caco2) cell line (Miller, 1997; Jin et al., 2014).
Fig. 3.
Microbial synthesis of AHR ligands and host metabolism of indole.
The EC50 of IAA and tryptamine were determined to be 0.5 mM and 0.2 mM, respectively, using a [3H] TCDD competition assay, indicating that they bind to the ligand-binding domain with low affinity (Heath-Pagliuso et al., 1998). The monoamine alkaloid tryptamine is derived from the direct decarboxylation of tryptophan. Tryptophan is converted to IAA via the enzymes tryptophan monooxygenase and indole-3-acetamide hydrolase, which constitute the indole-3-acetamide pathway (Tsavkelova et al., 2012). The common fecal metabolite 3-methyl indole (skatole), generated by decarboxylation of IAA, has been shown to facilitate transcription of AHR target genes in human bronchial and colonic epithelial cells (Weems and Yost, 2010; Hubbard et al., 2015). The physiologic implications of AHR activation by tryptamine, IAA, or skatole remain to be established.
The catabolism of tryptophan by commensal lactobacilli has been shown to hinder colonization of the intestinal tract by pathogenic Candida albicans through AHR dependent expression of IL22 (Zelante et al., 2013). Targeted metabolomic analysis of Lactobacillus reuteri and L. johnsonii tryptophan catabolism has identified the indole derivative indole-3-aldehyde (Iald), which is generated through the indole pyruvate pathway, catalyzed by the enzyme aromatic amino acid aminotransferase. In vivo treatments with Iald were found to activate AHR and to promote intestinal homeostasis through induction of interleukin-22 (IL-22) (Zelante et al., 2013). The manipulation of pathways that promote Iald production has demonstrated therapeutic promise although more evidence is needed to address IL-22–mediated activity in human models. It will be important to determine whether normal colonization of lactobacillus species can generate adequate amounts of ligand to activate AHR.
Bacterial metabolism of tryptophan by the tryptophanase pathway has been shown to generate indole and pyruvate (Fig. 3). Indole is shuttled in and out of bacteria by passive diffusion or active transport by AcrEF-TolC and Mtr transporters (Yanofsky et al., 1991; Kawamura-Sato et al., 1999; Pinero-Fernandez et al., 2011). Interspecies signaling by indole regulates many physiologic functions, such as biofilm formation, motility, plasmid stability, virulence, and antibiotic resistance in neighboring microflora (Bansal et al., 2007; Lee et al., 2007; Lee and Lee, 2010). Metabolic analysis of human fecal samples has detected indole at concentrations of 250–1100 μM (Karlin et al., 1985; Zuccato et al., 1993). Such high in vivo concentrations of the metabolite suggest that it may facilitate a mutually beneficial relationship with the host. In fact, treatment of human intestinal epithelial cells (HCT-8) with indole was shown to increase barrier function and attenuate inflammatory markers (Bansal et al., 2010).
In recent competitive ligand-binding experiments indole was found to preferentially activate human AHR compared with mouse AHR (Miller, 1997; Hubbard et al., 2015). In addition, indole was able to induce DRE-dependent luciferase activity with EC50 of ∼3 μM, which is well within the range of relevant physiologic concentrations. The physiologic significance of microbial indole generation upon the intestinal epithelium and its effect upon basal AHR signaling remain to be established in in vivo models.
A lack of intestinal homeostasis has been shown to be a contributing factor in the pathology of many disorders, including inflammatory bowel disease, Crohn’s disease, ulcerative colitis, diabetes, obesity, and cancer. Maintenance of intestinal homeostasis solely by dietary AHR ligands would be transient and would vary greatly according to the types of ligands consumed, their respective bioavailability, and their half-lives. Thus, synthesis of putative AHR ligands such as indole by the intestinal commensal microflora may provide a more consistent source of in vivo AHR stimulation. Such AHR stimulation arising from microbial activity may represent a mechanism by which AHR acts as a sensor of the microbiota community and, through its established role as a modulator of immune function, maintains host-microbe homeostasis. Clearly, the implications of microbial ligand synthesis and its effect upon intestinal disease require further investigation.
Host Metabolism of Indoles
Prevailing evidence suggests that the production of indole is dependent upon microbial metabolic activity (Wikoff et al., 2009). Subsequent absorption of indole by the host occurs via passive diffusion through the colonic epithelia. Hepatic uptake and metabolism of indole is another likely source for the generation of potent AHR ligands (Fig. 3). For example, phase I metabolism of indole by cytochrome P450 2E1 (Cyp2e1) in rat liver microsomes catalyzes the hydroxylation of indole to form 3-hydroxy-indole (indoxyl) and isatin. In addition, recombinant human cytochrome P450 enzymes within yeast expression systems can also catalyze the same reaction (Gillam et al., 2000; Banoglu et al., 2001).
In vivo generation of the indigoids indigo and indirubin have been proposed to occur through radical redox mechanisms involving the unstable intermediates indoxyl and isatin (Gillam et al., 2000). Indigo and indirubin have been identified in human urine and found to be potent activators of AHR (Adachi et al., 2001; Peter Guengerich et al., 2004; Sugihara et al., 2004). Indirubin was found to be a more potent human AHR agonist when compared with the murine Ahb-1 allele (high affinity for TCDD) of the receptor (Flaveny et al., 2009). Furthermore, in a yeast DRE-driven β-galactosidase reporter expressing human AHR, indirubin was found to possess an EC50 of ∼0.2 nM, 45-fold less than TCDD (Adachi et al., 2001). Whether the liver synthesizes indirubin or indigo in quantities sufficient to activate AHR has not been established.
Hepatic phase II drug metabolism of indoxyl via sulfation by sulfotransferases generates the uremic toxin indoxyl-3-sulfate (I3S). I3S is recognized as a human AHR agonist with 500-fold greater potency in human versus murine hepatoma cell lines (Schroeder et al., 2010). Increased serum concentrations of I3S have been correlated with vascular dysfunction in chronic kidney disease (CKD) patients (Barreto et al., 2009). Assessment of serum from dialysis patients indicates an accumulation of I3S to high micromolar concentrations. Failed excretion and subsequent accumulation of I3S may contribute to the overt toxicity seen in dialysis patients and has been shown to contribute to increased risk of tumor growth (Wong et al., 2013). Antagonism of AHR or targeted inhibition of I3S synthesis may have a beneficial therapeutic effect in CKD patients (Sallee et al., 2014).
Oxidative stress from the accumulation of I3S has also been shown to increase the risk of cardiovascular disease in CDK patients through oxidative modification of low-density lipoproteins (Cao et al., 2015). The in vitro oxidation of low-density lipoproteins by indoxyl radicals has been shown to generate the AHR activator tryptanthrin (Praschberger et al., 2014). Tryptanthrin synthesis has also been characterized previously in Candida lipolytica and Malassezia yeast cultures through the catabolism of tryptophan (Schrenk et al., 1999; Vlachos et al., 2012). Furthermore, the production of AHR agonists within the epidermis by Malassezia yeasts have been shown to contribute to seborrheic dermatitis disease progression (Gaitanis et al., 2008). However, AHR activity mediated by tryptanthrin appears to be localized to the skin, limiting its in vivo relevance as an endogenous regulator.
The metabolic profiling of tissue extracts has led to the identification of endogenous AHR ligands that contain indolyl moieties. Organic extractions of porcine lung tissue led to the discovery of the AHR ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), which was shown to be a direct high-affinity ligand for human, mouse, and fish receptors (Song et al., 2002). However, the presence of ITE in vivo has not been established, and thus it is possible that the generation ITE may be an artifact of the extraction process in which the methanol extract was heated to high temperatures, as described in the initial report.
Nevertheless, in mouse models of experimental autoimmune uveitis, ITE was found to inhibit the onset of symptoms through an AHR-dependent mechanism of Treg differentiation (Nugent et al., 2013). ITE has also been shown to possess AHR-independent biologic activity, such as the inhibition of transforming growth factor-β1–mediated myofibroblast differentiation, which prevents fibrosis within human tissue (Lehmann et al., 2011). The AHR ligand ITE has many biologic activities, but its classification as an “endogenously” derived compound remains to be established.
Host Metabolism of Tryptophan
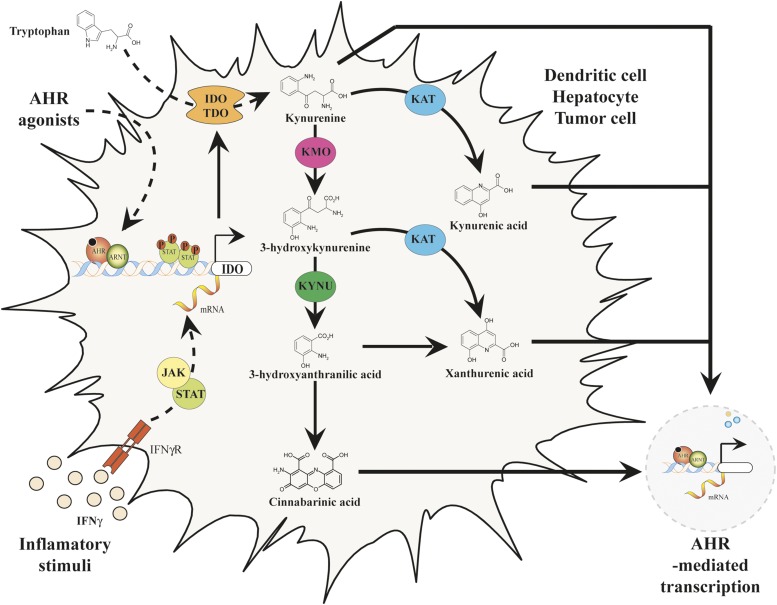
Over the past century, biochemical analyses found that the enzymatic reactions that account for greater than 90% of the peripheral metabolism of tryptophan in mammals reside in the kynurenine pathway (Leklem, 1971). The kynurenine pathway is composed of multiple enzymatic steps that facilitate the metabolism of tryptophan to nicotinamide adenine dinucleotide and a number of bioactive intermediate byproducts, some of which have been characterized as activators of AHR (Fig. 4) (Bohar et al., 2015). In addition, the kynurenine pathway is thought to play a key role in cancer immunity through signaling cascades that promote immune suppression or tolerance.
Fig. 4.
Endogenous tryptophan metabolism through the kynurenine pathway generates AHR ligands.
The depletion of tryptophan and subsequent generation of ligands could activate AHR, which can alter naive CD4+ T-helper cell differentiation pathways to favor an anti-inflammatory Treg rather than a TH17 phenotype (Quintana et al., 2008; Veldhoen et al., 2008). This alteration in adaptive immunity functions to hinder immune surveillance within the tumor microenvironment. Increased expression of the enzymes in this pathway has been observed in tumors, as evidenced by an increase in the kynurenine/tryptophan ratio, which has been shown to positively correlate with cancer progression (Suzuki et al., 2010; Pilotte et al., 2012).
The kynurenine pathway is initiated by host metabolism of tryptophan via indoleamine-2,3-dioxygenase (IDO1) and tryptophan-2,3-dioxygenase (TDO2). TDO2 and IDO1 are analogous enzymes that perform an identical catabolic conversion of tryptophan to kynurenine but are structurally divergent and exhibit distinct expression patterns in vivo (Ball et al., 2014). Interferon-γ signaling has been shown to regulate IDO1 expression via activation of a Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling in cell types of epithelial or monocytic lineage (Yoshida et al., 1981; Nguyen et al., 2010). IDO1 induction was also found to depend on AHR transcriptional regulation within bone-marrow derived dendritic cells (Nguyen et al., 2010).
More recent studies have identified TDO2 enzyme activity as an additional mechanism of tryptophan depletion mainly in hepatocytes and neurons. The up-regulated expression of TDO2 in glioblastoma tissues has displayed a positive correlation with the induction of the AHR target gene CYP1B1, which supports the hypothesis of AHR ligand generation within the kynurenine pathway (Opitz et al., 2011). The metabolism of tryptophan by IDO/TDO in glioma cell lines yields significant amounts of kynurenine, which was found to be a low-affinity ligand for AHR (Mezrich et al., 2010; Opitz et al., 2011). Additional metabolism of kynurenine leads to the generation of more potent AHR ligands, which may have an increased impact upon immune regulation and tumor malignancy.
The metabolite kynurenic acid (KA), generated by the irreversible transamination of kynurenine by kynurenine aminotransferases, was found to be a relatively potent endogenous ligand for human AHR (Han et al., 2009; DiNatale et al., 2010a). Interestingly, KA can be detected in serum at a concentration of ∼5 μM in some CKD patients (Schefold et al., 2009). KA at a physiologically relevant dose of 100 nM mediated activation of AHR and was found to induce prototypical AHR target genes and synergistically increase the expression of IL6 in the presence of inflammatory signaling (DiNatale et al., 2010a). This investigation also found that the kynurenine metabolite xanthurenic acid displayed agonist potential in an AHR-dependent luciferase reporter in human hepatoma cells. Xanthurenic acid is generated by transamination of the kynurenine metabolite 3-hydroxykynurenine.
The kynurenine metabolite cinnabarinic acid (CA) has been identified as an additional endogenous AHR agonist that mediates AHR-dependent transcription of IL22 in human and murine CD4+ T cells (Lowe et al., 2014). CA is generated by condensation of 3-hydroxyanthranilic acid, which is produced by kynureninase from 3-hydroxykynurenine (Fig. 4). Recently, CA also has been shown to play a cytoprotective role in endoplasmic reticulum or oxidative stress–induced apoptosis through AHR-dependent induction of stanniocalcin 2 (Stc2) (Joshi et al., 2015). Further investigation of the kynurenine pathway may lead to the identification of additional endogenous AHR ligands.
The identity of the metabolite(s) primarily responsible for AHR activation in tumor tissue has not yet been confirmed. Increased levels of AHR activity in tumors, characterized by increased CYP1B1 mRNA expression, have been shown to positively correlate with poor survival in glioma patients (Opitz et al., 2011). In addition, studies examining metabolite concentrations in pancreatic adenocarcinomas identified a positive correlation between KA levels and tumor size (Botwinick et al., 2014). Such evidence supports the notion that AHR stimulation may increase tumor aggressiveness through metabolites of the kynurenine pathway. Targeted antagonism of AHR or the kynurenine pathway enzymes responsible for synthesis of higher affinity ligands may be of therapeutic interest as a supplement to traditional chemotherapy and is worthy of exploration. Indeed, pharmaceutic inhibition of IDO function is currently in the initial stages of clinical trials.
Photo-Oxidation of Tryptophan
Studies have found that exposure of culture media to UV light leads to increased AHR activation and induction of cytochrome P450 target genes within cultured cells (Oberg et al., 2005). The photo-oxidation of tryptophan was found to generate 6-formylindolo[3,2-b]carbazole (FICZ), which has been established as a potent AHR ligand with an affinity similar to that of TCDD (Fig. 5) (Rannug et al., 1987; Rannug et al., 1995). FICZ treatment of a murine autoimmune encephalitis model displayed an increase in the severity of symptoms, contradictory to that observed with TCDD (Quintana et al., 2008; Veldhoen et al., 2008). Further investigation found that FICZ induced differentiation of TH17 cells and expression of proinflammatory Il17 rather than anti-inflammatory Treg cells. This unique physiologic activity of FICZ suggests that it may exhibit selective AHR activity relative to other AHR ligands.
Fig. 5.
Summary of endogenous pathways of AHR ligand synthesis. (1) Microbial production of indole, (2) microbial/host indole metabolism to generate AHR ligands, (3) tryptophan UV photo-oxidation to form 6-formylindolo[3,2-b]carbazole (FICZ), (4) endogenous tryptophan metabolism via the kynurenine pathway, and (5) diet-derived ligand synthesis are all pathways of AHR ligand synthesis that mediate (6) AHR activation and transcription of target genes interleukin-6 (IL6), cytochrome P450 1A1 (CYP1A1), vascular endothelial growth factor A (VEGFA), prostaglandin G/H synthase 2 (PTGS2), interleukin-22 (IL22), and cytochrome P450 1B1 (CYP1B1).
The relevance of FICZ in in vivo systems remains to be firmly established. Epidermal UV irradiation of both human skin or keratinocyte cultures was found to increase AHR target monooxygenases, but this apparently did not depend on FICZ formation (Katiyar et al., 2000; Gonzalez et al., 2001). The metabolic analysis of human urine has indicated the presence of multiple FICZ conjugates, suggesting it may be an endogenous high-affinity regulator of AHR (Wincent et al., 2009). However, confirmation of subdermal synthesis of FICZ has not yet occurred.
Studies in mouse hepatoma cells indicate a negative feedback loop in which FICZ induces Cyp1a1 expression, which in turn leads to its rapid degradation (Wei et al., 2000). Thus, limited in vivo synthesis and rapid degradation make FICZ unlikely to be a significant contributor to AHR-mediated activity within the context of an entire organism. Nevertheless, FICZ may have significant implications within the context of AHR activation in skin, where constant UV exposure may generate sufficient quantities of the ligand to activate AHR.
Human versus Mouse AHR Ligand Selectivity
The identity of the primary mediators of AHR activity remains elusive due to the ability of the receptor to bind a number of different classes of compounds. This issue is further complicated when trying to determine which endogenous ligands may have important implications in human health. Although the regulation of monooxygenase expression by AHR appears to be conserved, there exist a number of structural distinctions between murine and human isoforms of the receptor.
Amino acid sequence comparisons between the human and murine receptors (Ahb-1 allele) reveal 85% sequence homology in the amino terminal half of the receptor that contains the ligand-binding domain. These variations lead to significant differences in ligand affinity and selectivity between species. For example, mouse AHR (Ahb-1) displays a 10-fold increased affinity for TCDD relative to human AHR. This difference in affinity for dioxin has been shown to be associated with a single amino acid substitution in the ligand-binding pocket (Ema et al., 1994; Ramadoss and Perdew, 2004). The human receptor has been shown to have increased affinity for indole-based derivatives such as indigoids, indole, indoxyl-sulfate, and KA (Flaveny et al., 2009; DiNatale et al., 2010a; Schroeder et al., 2010; Hubbard et al., 2015). Enhanced affinity of the human receptor for particular compounds may indicate an evolutionary adaptation to respond to specific endogenous and exogenous regulators that mediate certain physiologic responses (e.g., during inflammation).
Considering the differential ligand affinity frequently observed between species, the primary use of in vitro or in vivo mouse systems to catalog potential ligands of AHR will not always identify ligands that bind to AHR in other species. For this reason, both human and murine systems should be used when trying to assess the putative AHR ligand potential of a given compound.
Although functionally conserved, mouse and human AHR exhibit significant differences in their ability to modulate gene expression. The expression of human AHR in a transgenic mouse model allowed the assessment of whether human AHR exhibits similar transcriptional activity relative to mouse AHR in mouse hepatocytes (Flaveny et al., 2010). Although many of the AHR-dependent targets are conserved, species-specific targets do exist that appear to be due to inherent differences in AHR structure.
Clearly, when comparing two species other differences in transcriptional regulation are also likely, such as where functional DRE are found in various promoter regions. Species-specific differences in AHR activity indicate the need for a suitable transgenic mouse model that possesses tissue-specific or global knock-in of human AHR at the murine locus. Such humanized AHR models may facilitate the in vivo analysis of putative endogenous AHR ligands and allow for a greater understanding of the impact of endogenous signaling in human health and disease.
Possible Mechanism of AHR Activation by Low-Molecular-Weight Compounds
Exogenous high-affinity AHR ligands such as halogenated dioxins and dibenzofurans are products of the combustion of organic matter and industrial activity. Such ligands generally are not metabolically labile but are highly lipophilic, thus allowing bioaccumulation. The accumulation of these ligands, combined with their high affinity for AHR and subsequent persistent modulation of AHR-dependent signaling, contribute significantly to AHR-associated toxicity. However, lower endogenous levels of activation of AHR have been shown be beneficial in the maintenance of immune health and intestinal homeostasis.
The adaptation of AHR to bind endogenous compounds with moderate to low affinity may have increased its physiologic relevance. Such ligands may not structurally resemble dioxin, and they are significantly smaller in size than polycyclic molecules. AHR displays decreased affinity for ligands such as indole, KA, or indoxyl sulfate, relative to TCDD. The accommodation and interaction of small molecules within the ligand-binding domain of AHR is likely to differ from that observed with high-affinity ligands such as dioxin (Backlund and Ingelman-Sundberg, 2004). Indeed, recent studies provide evidence that human AHR has the capacity to bind two indole molecules per ligand-binding pocket to facilitate activation (Hubbard et al., 2015).
The concept of small-molecule activation or combinatorial activation of AHR by two molecules represents a paradigm shift in AHR ligand-binding dynamics and expands the pool of potential ligands. The structural diversity of endogenous ligands may induce differential conformations of the ligand-bound receptor that ultimately dictate varying biologic activities (Lees and Whitelaw, 1999; Kronenberg et al., 2000; Patel et al., 2009; Murray et al., 2010a,b; Jin et al., 2012). Synthesis or accumulation of distinct endogenous ligands within certain cell types or tissues could mediate the tissue/cell selective activity of AHR. The lack of an x-ray crystal structure for AHR hinders efforts to better understand the ligand-receptor molecular interactions that mediate agonist, antagonist, and selective modulator activities (Safe and McDougal, 2002).
Summary
There are many routes of endogenous AHR ligand generation (Fig. 5), and all likely contribute in some way to modulating in vivo AHR function, with perhaps additional pathways and ligands yet to be identified. Considering the growing list of endogenous ligands that have been identified, it is improbable that a single bona fide endogenous ligand exists that is primarily responsible for mediating the in vivo physiologic functions of AHR. The basal activity of AHR within an organism may also be influenced by antagonists or selective modulators generated endogenously that have not yet been characterized. A more comprehensive and mechanistic understanding of all factors that influence AHR signaling pathways within an organism is critical to elucidating the physiologic function(s) of this fascinating receptor.
Acknowledgments
The authors thank Marcia H. Perdew and Kayla Smith for excellent editorial assistance.
Abbreviations
- AHR
aryl hydrocarbon receptor, ARNT, aryl hydrocarbon receptor nuclear translocator
- CA
cinnabarinic acid
- CKD
chronic kidney disease
- Cyp
cytochrome P450 enzyme, DRE, dioxin-response element
- DSS
dextran sodium sulfate
- FICZ
6-formylindolo[3,2-b]carbazole
- I3ACN
indole-3-acetonitrile
- I3C
indole-3-carbinol
- I3S
indoxyl-3-sulfate
- IAA
indole-3-acetic acid
- Iald
indole-3-aldehyde
- ICZ
indolo[3,2-b]carbazole
- IDO1
indoleamine-2,3-dioxygenase
- IL
interleukin
- ITE
2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester
- KA
kynurenic acid
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TDO2
tryptophan-2,3-dioxygenase
- Treg
regulatory T cells
Authorship Contributions
Participated in research design: Hubbard, Murray, Perdew.
Performed data analysis: Hubbard, Murray, Perdew.
Wrote or contributed to the writing of the manuscript: Hubbard, Murray, Perdew.
Footnotes
This research was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grants ES004869, ES019964 (to G.H.P.)].
References
- Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, Miller CA, 3rd, Kato T, Saeki K, Matsuda T. (2001) Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem 276:31475–31478. [DOI] [PubMed] [Google Scholar]
- Arsenescu R, Arsenescu V, Zhong J, Nasser M, Melinte R, Dingle RW, Swanson H, de Villiers WJ. (2011) Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm Bowel Dis 17:1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund M, Ingelman-Sundberg M. (2004) Different structural requirements of the ligand binding domain of the aryl hydrocarbon receptor for high- and low-affinity ligand binding and receptor activation. Mol Pharmacol 65:416–425. [DOI] [PubMed] [Google Scholar]
- Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. (2014) Tryptophan-catabolizing enzymes—party of three. Front Immunol 5:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banoglu E, Jha GG, King RS. (2001) Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur J Drug Metab Pharmacokinet 26:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T, Alaniz RC, Wood TK, Jayaraman A. (2010) The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA 107:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal T, Englert D, Lee J, Hegde M, Wood TK, Jayaraman A. (2007) Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun 75:4597–4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) (2009) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM. (2011) Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci 120:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. (2013) bHLH-PAS proteins in cancer. Nat Rev Cancer 13:827–841. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Conolly RB, Kaminski NE, Thomas RS, Andersen ME, Zhang Q. (2010) A bistable switch underlying B-cell differentiation and its disruption by the environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci 115:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittinger MA, Nguyen LP, Bradfield CA. (2003) Aspartate aminotransferase generates proagonists of the aryl hydrocarbon receptor. Mol Pharmacol 64:550–556. [DOI] [PubMed] [Google Scholar]
- Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. (1991) Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci USA 88:9543–9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohár Z, Toldi J, Fülöp F, Vécsei L. (2015) Changing the face of kynurenines and neurotoxicity: therapeutic considerations. Int J Mol Sci 16:9772–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botwinick IC, Pursell L, Yu G, Cooper T, Mann JJ, Chabot JA. (2014) A biological basis for depression in pancreatic cancer. HPB (Oxford) 16:740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield CA, Bjeldanes LF. (1987) Structure-activity relationships of dietary indoles: a proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health 21:311–323. [DOI] [PubMed] [Google Scholar]
- Bradlow HL, Michnovicz J, Telang NT, Osborne MP. (1991) Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis 12:1571–1574. [DOI] [PubMed] [Google Scholar]
- Cao XS, Chen J, Zou JZ, Zhong YH, Teng J, Ji J, Chen ZW, Liu ZH, Shen B, Nie YX, et al. (2015) Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clin J Am Soc Nephrol 10:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaro CR, Patel RD, Marcus CB, Perdew GH. (2007) Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol 72:1369–1379. [DOI] [PubMed] [Google Scholar]
- Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. (2001) Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 20:2927–2936. [DOI] [PubMed] [Google Scholar]
- Chung KT, Gadupudi GS. (2011) Possible roles of excess tryptophan metabolites in cancer. Environ Mol Mutagen 52:81–104. [DOI] [PubMed] [Google Scholar]
- Denison MS, Fisher JM, Whitlock JP., Jr (1988) Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci USA 85:2528–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43:309–334. [DOI] [PubMed] [Google Scholar]
- DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. (2010a) Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 115:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Schroeder JC, Francey LJ, Kusnadi A, Perdew GH. (2010b) Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J Biol Chem 285:24388–24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNatale BC, Smith K, John K, Krishnegowda G, Amin SG, Perdew GH. (2012) Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol Cancer Res 10:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosz EB, Ku KM, Juvik JA, Jeffery EH. (2014) Total myrosinase activity estimates in brassica vegetable produce. J Agric Food Chem 62:8094–8100. [DOI] [PubMed] [Google Scholar]
- Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fujii-Kuriyama Y. (1994) Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J Biol Chem 269:27337–27343. [PubMed] [Google Scholar]
- Exon JH, South EH, Magnuson BA, Hendrix K. (2001) Effects of indole-3-carbinol on immune responses, aberrant crypt foci, and colonic crypt cell proliferation in rats. J Toxicol Environ Health A 62:561–573. [DOI] [PubMed] [Google Scholar]
- Fares FA. (2014) The anti-carcinogenic effect of indole-3-carbinol and 3,3′-diindolylmethane and their mechanism of action. Med Chem S1:002 DOI: 10.4172/2161-0444.S1-002. [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. (1995) Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 268:722–726. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. (1997) Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 34:605–614. [DOI] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA, Chiaro CR, Perdew GH. (2009) Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol 75:1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA, Perdew GH. (2010) Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicol Sci 114:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatake CJ, Marshall NB, Kerkvliet NI. (2008) 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation of alloreactive CD8+ T cells toward a regulatory T cell phenotype by a mechanism that is dependent on aryl hydrocarbon receptor in CD4+ T cells. J Immunotoxicol 5:81–91. [DOI] [PubMed] [Google Scholar]
- Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. (2005) Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol 175:4184–4188. [DOI] [PubMed] [Google Scholar]
- Gaitanis G, Magiatis P, Stathopoulou K, Bassukas ID, Alexopoulos EC, Velegraki A, Skaltsounis AL. (2008) AhR ligands, malassezin, and indolo[3,2-b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. J Invest Dermatol 128:1620–1625. [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Henry EC, Collins LL. (2008) Expression and activity of aryl hydrocarbon receptors in development and cancer. Crit Rev Eukaryot Gene Expr 18:279–321. [DOI] [PubMed] [Google Scholar]
- Gillam EM, Notley LM, Cai H, De Voss JJ, Guengerich FP. (2000) Oxidation of indole by cytochrome P450 enzymes. Biochemistry 39:13817–13824. [DOI] [PubMed] [Google Scholar]
- Gonzalez MC, Marteau C, Franchi J, Migliore-Samour D. (2001) Cytochrome P450 4A11 expression in human keratinocytes: effects of ultraviolet irradiation. Br J Dermatol 145:749–757. [DOI] [PubMed] [Google Scholar]
- Han Q, Robinson H, Cai T, Tagle DA, Li J. (2009) Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol Cell Biol 29:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. (1998) Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 37:11508–11515. [DOI] [PubMed] [Google Scholar]
- Huang Z, Jiang Y, Yang Y, Shao J, Sun X, Chen J, Dong L, Zhang J. (2013) 3,3′-Diindolylmethane alleviates oxazolone-induced colitis through Th2/Th17 suppression and Treg induction. Mol Immunol 53:335–344. [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG, Patterson AD, Perdew GH. (2015) Adaptation of the aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 5, 12689; 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. (1998) Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem 273:2895–2904. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Tachibana T, Watanabe J, Yoshida M, Yoneda Y, Kawajiri K. (2000) Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J Biochem 127:503–509. [DOI] [PubMed] [Google Scholar]
- Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. (2007) Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest 117:1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. (1993) Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol 45:1129–1136. [DOI] [PubMed] [Google Scholar]
- Jeuken A, Keser BJ, Khan E, Brouwer A, Koeman J, Denison MS. (2003) Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J Agric Food Chem 51:5478–5487. [DOI] [PubMed] [Google Scholar]
- Jin UH, Lee SO, Safe S. (2012) Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J Pharmacol Exp Ther 343:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. (2014) Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 85:777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Carter DE, Harper TA, Elferink CJ. (2015) Aryl hydrocarbon receptor-dependent stanniocalcin 2 induction by cinnabarinic acid provides cytoprotection against endoplasmic reticulum and oxidative stress. J Pharmacol Exp Ther 353:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin DA, Mastromarino AJ, Jones RD, Stroehlein JR, Lorentz O. (1985) Fecal skatole and indole and breath methane and hydrogen in patients with large bowel polyps or cancer. J Cancer Res Clin Oncol 109:135–141. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Matsui MS, Mukhtar H. (2000) Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol 114:328–333. [DOI] [PubMed] [Google Scholar]
- Kawamura-Sato K, Shibayama K, Horii T, Iimuma Y, Arakawa Y, Ohta M. (1999) Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol Lett 179:345–352. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Poellinger L, Pongratz I. (1999) Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J Biol Chem 274:13519–13524. [DOI] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. (2011) Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334:1561–1565. [DOI] [PubMed] [Google Scholar]
- Kojima T, Tanaka T, Mori H. (1994) Chemoprevention of spontaneous endometrial cancer in female Donryu rats by dietary indole-3-carbinol. Cancer Res 54:1446–1449. [PubMed] [Google Scholar]
- Kronenberg S, Esser C, Carlberg C. (2000) An aryl hydrocarbon receptor conformation acts as the functional core of nuclear dioxin signaling. Nucleic Acids Res 28:2286–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoti TS, John K, Hughes JM, Kusnadi A, Murray IA, Krishnegowda G, Amin S, Perdew GH. (2013) Aryl hydrocarbon receptor antagonism mitigates cytokine-mediated inflammatory signalling in primary human fibroblast-like synoviocytes. Ann Rheum Dis 72:1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, Bradfield CA. (2005) The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol 67:714–720. [DOI] [PubMed] [Google Scholar]
- Lee J, Jayaraman A, Wood TK. (2007) Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee J. (2010) Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444. [DOI] [PubMed] [Google Scholar]
- Lees MJ, Whitelaw ML. (1999) Multiple roles of ligand in transforming the dioxin receptor to an active basic helix-loop-helix/PAS transcription factor complex with the nuclear protein Arnt. Mol Cell Biol 19:5811–5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann GM, Xi X, Kulkarni AA, Olsen KC, Pollock SJ, Baglole CJ, Gupta S, Casey AE, Huxlin KR, Sime PJ, et al. (2011) The aryl hydrocarbon receptor ligand ITE inhibits TGFβ1-induced human myofibroblast differentiation. Am J Pathol 178:1556–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leklem JE. (1971) Quantitative aspects of tryptophan metabolism in humans and other species: a review. Am J Clin Nutr 24:659–672. [DOI] [PubMed] [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147:629–640. [DOI] [PubMed] [Google Scholar]
- Loub WD, Wattenberg LW, Davis DW. (1975) Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst 54:985–988. [PubMed] [Google Scholar]
- Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, Wang C, Patel G, Franks DG, Schlezinger J, et al. (2014) Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS ONE 9:e87877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luang-In V, Narbad A, Nueno-Palop C, Mithen R, Bennett M, Rossiter JT. (2014) The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol Nutr Food Res 58:875–883. [DOI] [PubMed] [Google Scholar]
- Marconett CN, Sundar SN, Poindexter KM, Stueve TR, Bjeldanes LF, Firestone GL. (2010) Indole-3-carbinol triggers aryl hydrocarbon receptor-dependent estrogen receptor (ER)alpha protein degradation in breast cancer cells disrupting an ERalpha-GATA3 transcriptional cross-regulatory loop. Mol Biol Cell 21:1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. (1998) Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol 18:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185:3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michnovicz JJ, Bradlow HL. (1990) Induction of estradiol metabolism by dietary indole-3-carbinol in humans. J Natl Cancer Inst 82:947–949. [DOI] [PubMed] [Google Scholar]
- Miller CA., 3rd (1997) Expression of the human aryl hydrocarbon receptor complex in yeast. Activation of transcription by indole compounds. J Biol Chem 272:32824–32829. [DOI] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, et al. (1997) Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 2:645–654. [DOI] [PubMed] [Google Scholar]
- Mohinta S, Kannan AK, Gowda K, Amin SG, Perdew GH, August A. (2015) Differential regulation of th17 and T regulatory cell differentiation by aryl hydrocarbon receptor dependent xenobiotic response element dependent and independent pathways. Toxicol Sci 145:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte J, Abreu AC, Borges A, Simões LC, Simões M. (2014) Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens 3:473–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, MacDonald TT, Pallone F, Monteleone G. (2011) Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141:237–248, e1. [DOI] [PubMed] [Google Scholar]
- Murray IA, Krishnegowda G, DiNatale BC, Flaveny C, Chiaro C, Lin JM, Sharma AK, Amin S, Perdew GH. (2010a) Development of a selective modulator of aryl hydrocarbon (Ah) receptor activity that exhibits anti-inflammatory properties. Chem Res Toxicol 23:955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IA, Morales JL, Flaveny CA, Dinatale BC, Chiaro C, Gowdahalli K, Amin S, Perdew GH. (2010b) Evidence for ligand-mediated selective modulation of aryl hydrocarbon receptor activity. Mol Pharmacol 77:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IA, Patterson AD, Perdew GH. (2014) Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer 14:801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshon-Kedmi M, Yannai S, Haj A, Fares FA. (2003) Indole-3-carbinol and 3,3′-diindolylmethane induce apoptosis in human prostate cancer cells. Food Chem Toxicol 41:745–752. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (NTP) (2014) Toxicology studies of indole-3-carbinol in F344/N rats and B6C3F1/N mice and toxicology and carcinogenesis studies of indole-3-carbinol in Harlan Sprague Dawley rats and B6C3F1/N mice (Gavage Studies), National Institutes of Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. (2008) The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. (2010) Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA 107:19961–19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent LF, Shi G, Vistica BP, Ogbeifun O, Hinshaw SJ, Gery I. (2013) ITE, a novel endogenous nontoxic aryl hydrocarbon receptor ligand, efficiently suppresses EAU and T-cell-mediated immunity. Invest Ophthalmol Vis Sci 54:7463–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukaya M, Moran S, Bradfield CA. (2009) The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc Natl Acad Sci USA 106:4923–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg M, Bergander L, Håkansson H, Rannug U, Rannug A. (2005) Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci 85:935–943. [DOI] [PubMed] [Google Scholar]
- Oganesian A, Hendricks JD, Pereira CB, Orner GA, Bailey GS, Williams DE. (1999) Potency of dietary indole-3-carbinol as a promoter of aflatoxin B1-initiated hepatocarcinogenesis: results from a 9000 animal tumor study. Carcinogenesis 20:453–458. [DOI] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203. [DOI] [PubMed] [Google Scholar]
- Patel RD, Murray IA, Flaveny CA, Kusnadi A, Perdew GH. (2009) Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest 89:695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH. (1988) Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem 263:13802–13805. [PubMed] [Google Scholar]
- Peter Guengerich F, Martin MV, McCormick WA, Nguyen LP, Glover E, Bradfield CA. (2004) Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch Biochem Biophys 423:309–316. [DOI] [PubMed] [Google Scholar]
- Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frédérick R, De Plaen E, Uyttenhove C, Wouters J, Masereel B, et al. (2012) Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA 109:2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñero-Fernandez S, Chimerel C, Keyser UF, Summers DK. (2011) Indole transport across Escherichia coli membranes. J Bacteriol 193:1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland A, Knutson JC. (1982) 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol 22:517–554. [DOI] [PubMed] [Google Scholar]
- Praschberger M, Hermann M, Wanner J, Jirovetz L, Exner M, Kapiotis S, Gmeiner BM, Laggner H. (2014) The uremic toxin indoxyl sulfate acts as a pro- or antioxidant on LDL oxidation. Free Radic Res 48:641–648. [DOI] [PubMed] [Google Scholar]
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. (2012) The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. (2008) Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71. [DOI] [PubMed] [Google Scholar]
- Ramadoss P, Perdew GH. (2004) Use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin as a probe to determine the relative ligand affinity of human versus mouse aryl hydrocarbon receptor in cultured cells. Mol Pharmacol 66:129–136. [DOI] [PubMed] [Google Scholar]
- Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafström AK. (1987) Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem 262:15422–15427. [PubMed] [Google Scholar]
- Rannug U, Rannug A, Sjöberg U, Li H, Westerholm R, Bergman J. (1995) Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem Biol 2:841–845. [DOI] [PubMed] [Google Scholar]
- Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. (2005) A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev 14:1953–1960. [DOI] [PubMed] [Google Scholar]
- Reyes H, Reisz-Porszasz S, Hankinson O. (1992) Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 256:1193–1195. [DOI] [PubMed] [Google Scholar]
- Richmond O, Ghotbaddini M, Allen C, Walker A, Zahir S, Powell JB. (2014) The aryl hydrocarbon receptor is constitutively active in advanced prostate cancer cells. PLoS ONE 9:e95058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse M, Singh NP, Nagarkatti PS, Nagarkatti M. (2013) Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and Th17 cells. Br J Pharmacol 169:1305–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands JC, Gustafsson JA. (1997) Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol 27:109–134. [DOI] [PubMed] [Google Scholar]
- Safa M, Tavasoli B, Manafi R, Kiani F, Kashiri M, Ebrahimi S, Kazemi A. (2015) Indole-3-carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells. Tumour Biol 36:3919–3930. [DOI] [PubMed] [Google Scholar]
- Safe S, Lee SO, Jin UH. (2013) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci 135:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, McDougal A. (2002) Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers (review). Int J Oncol 20:1123–1128. [PubMed] [Google Scholar]
- Saito R, Miki Y, Hata S, Takagi K, Iida S, Oba Y, Ono K, Ishida T, Suzuki T, Ohuchi N, et al. (2014) Aryl hydrocarbon receptor in breast cancer—a newly defined prognostic marker. Horm Cancer 5:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. (2014) The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 6:934–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. (2004) Indole-3-carbinol and prostate cancer. J Nutr 134(12, Suppl)3493S–3498S. [DOI] [PubMed] [Google Scholar]
- Schefold JC, Zeden JP, Fotopoulou C, von Haehling S, Pschowski R, Hasper D, Volk HD, Schuett C, Reinke P. (2009) Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 24:1901–1908. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. (1996) Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA 93:6731–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk D, Riebniger D, Till M, Vetter S, Fiedler HP. (1999) Tryptanthrins and other tryptophan-derived agonists of the dioxin receptor. Adv Exp Med Biol 467:403–408. [DOI] [PubMed] [Google Scholar]
- Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, et al. (2010) The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. (2001) Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev 10:501–508. [PubMed] [Google Scholar]
- Shen ES, Whitlock JP., Jr. (1992) Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J Biol Chem 267:6815–6819. [PubMed] [Google Scholar]
- Singh SS, Hord NG, Perdew GH. (1996) Characterization of the activated form of the aryl hydrocarbon receptor in the nucleus of HeLa cells in the absence of exogenous ligand. Arch Biochem Biophys 329:47–55. [DOI] [PubMed] [Google Scholar]
- Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. (2002) A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA 99:14694–14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom DK, Postlind H, Tukey RH. (1992) Characterization of the rabbit CYP1A1 and CYP1A2 genes: developmental and dioxin-inducible expression of rabbit liver P4501A1 and P4501A2. Arch Biochem Biophys 294:707–716. [DOI] [PubMed] [Google Scholar]
- Su JM, Lin P, Chang H. (2013) Prognostic value of nuclear translocation of aryl hydrocarbon receptor for non-small cell lung cancer. Anticancer Res 33:3953–3961. [PubMed] [Google Scholar]
- Sugihara K, Kitamura S, Yamada T, Okayama T, Ohta S, Yamashita K, Yasuda M, Fujii-Kuriyama Y, Saeki K, Matsui S, et al. (2004) Aryl hydrocarbon receptor-mediated induction of microsomal drug-metabolizing enzyme activity by indirubin and indigo. Biochem Biophys Res Commun 318:571–578. [DOI] [PubMed] [Google Scholar]
- Sutter CH, Bodreddigari S, Campion C, Wible RS, Sutter TR. (2011) 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol Sci 124:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H, Chida K. (2010) Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 67:361–365. [DOI] [PubMed] [Google Scholar]
- Takamura T, Harama D, Fukumoto S, Nakamura Y, Shimokawa N, Ishimaru K, Ikegami S, Makino S, Kitamura M, Nakao A. (2011) Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol 89:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, Okumura K, Ogawa H, Kitamura M, Nakao A. (2010) Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol 88:685–689. [DOI] [PubMed] [Google Scholar]
- Terashima J, Tachikawa C, Kudo K, Habano W, Ozawa S. (2013) An aryl hydrocarbon receptor induces VEGF expression through ATF4 under glucose deprivation in HepG2. BMC Mol Biol 14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavkelova E, Oeser B, Oren-Young L, Israeli M, Sasson Y, Tudzynski B, Sharon A. (2012) Identification and functional characterization of indole-3-acetamide-mediated IAA biosynthesis in plant-associated Fusarium species. Fungal Genet Biol 49:48–57. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106–109. [DOI] [PubMed] [Google Scholar]
- Vlachos C, Schulte BM, Magiatis P, Adema GJ, Gaitanis G. (2012) Malassezia-derived indoles activate the aryl hydrocarbon receptor and inhibit Toll-like receptor-induced maturation in monocyte-derived dendritic cells. Br J Dermatol 167:496–505. [DOI] [PubMed] [Google Scholar]
- Weems JM, Yost GS. (2010) 3-Methylindole metabolites induce lung CYP1A1 and CYP2F1 enzymes by AhR and non-AhR mechanisms, respectively. Chem Res Toxicol 23:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YD, Bergander L, Rannug U, Rannug A. (2000) Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole. Arch Biochem Biophys 383:99–107. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106:3698–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. (2009) The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem 284:2690–2696. [DOI] [PubMed] [Google Scholar]
- Wong G, Turner RM, Chapman JR, Howell M, Lim WH, Webster AC, Craig JC. (2013) Time on dialysis and cancer risk after kidney transplantation. Transplantation 95:114–121. [DOI] [PubMed] [Google Scholar]
- Yang X, Solomon S, Fraser LR, Trombino AF, Liu D, Sonenshein GE, Hestermann EV, Sherr DH. (2008) Constitutive regulation of CYP1B1 by the aryl hydrocarbon receptor (AhR) in pre-malignant and malignant mammary tissue. J Cell Biochem 104:402–417. [DOI] [PubMed] [Google Scholar]
- Yanofsky C, Horn V, Gollnick P. (1991) Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J Bacteriol 173:6009–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Imanishi J, Oku T, Kishida T, Hayaishi O. (1981) Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci USA 78:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, et al. (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385. [DOI] [PubMed] [Google Scholar]
- Zhang L, Savas U, Alexander DL, Jefcoate CR. (1998) Characterization of the mouse Cyp1B1 gene. Identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. J Biol Chem 273:5174–5183. [DOI] [PubMed] [Google Scholar]
- Zuccato E, Venturi M, Di Leo G, Colombo L, Bertolo C, Doldi SB, Mussini E. (1993) Role of bile acids and metabolic activity of colonic bacteria in increased risk of colon cancer after cholecystectomy. Dig Dis Sci 38:514–519. [DOI] [PubMed] [Google Scholar]