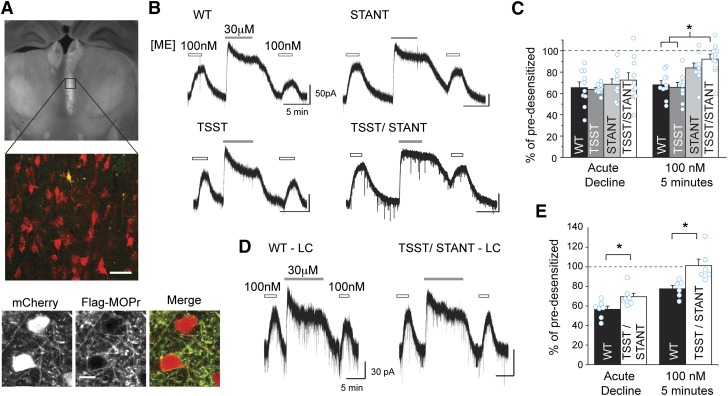
Fig. 5.
C-terminal serine and threonine residues are involved in sustained acute desensitization. (A) FLAG-MOPr + mCherry AAV2 was injected into the MD thalamus of MOPr KO mice. Coronal brain slices were made, and an mCherry expressing cell was filled with Alexa-488. The fixed coronal brain slice was imaged using widefield fluorescence (top) or laser scanning confocal microscopy (middle). Confocal image of an Alexa-488 (green) filled cell also expressing mCherry (red), (merged in yellow) among other AAV2 infected cells from the brain slice pictured above. Scale, 40 μm. Bottom: two-photon images of a live brain slice showing neurons in the MD thalamus expressing mCherry (left) and stained with M1-A488 anti-FLAG antibody (middle) and overlaid in the merged image (right). (Scale bars, 10 μm.) (B) Exemplary whole-cell electrophysiologic recordings from wild-type and mutant FLAG-MOPr expressing cells. Slices were treated with an approximately EC50 concentration of ME (100 nM). Cells were desensitized with ME (30 μM, 5 minutes) and then retested with EC50 ME 5 minutes later. (C) Summary data show the percentage of the remaining current following desensitization in the continued presence of ME 30 μM (acute decline) and the percentage amplitude of the EC50 ME measured 5 minutes after desensitization relative to the current evoked prior to desensitization (EC50, 5 minutes) in individual cells (open circles) and as an average (average ± S.E.M., n = 9–10 slices, 6–10 animals). (D) FLAG-MOPr + mCherry AAV2 was injected into the LC of MOPr KO mice, and representative whole-cell voltage-clamp recordings are shown for WT or TSST/STANT-7A MOPr. A subsaturating concentration of ME (100 nM) was applied before and following a 10-minute supersaturating concentration of ME (30 μM, 10 minutes) to induce acute desensitization. (E) Acute decline in the continued presence of agonist and sustained desensitization (EC50, 5 minutes) were measured as described above. Average and individual measurements (cyan open circles) are plotted (average ± S.E.M., n = 6–7 slices, 4–5 animals.). *P < 0.05 compared with WT, one-way ANOVA, Tukey’s post hoc.