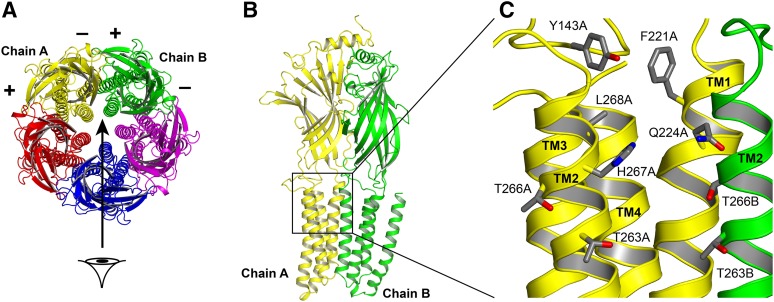
Fig. 1.
Structures of the GABAA receptor and the putative propofol binding pocket. (A) A top (extracellular) view of the receptor. The five subunits surround a centrally located pore. By convention, the subunits have “+” and “−” sides that are ordered in the fashion shown in the figure. The putative propofol binding site is located at the interface between two neighboring subunits. In β3 homomeric receptors, there are five identical β–β interfaces. Heteromeric α1β3 receptors likely have a stoichiometry of two α and three β subunits, and assemble as β–β–α–β–α (top view, counterclockwise). These receptors contain two β–α interfaces, two α–β interfaces, and one β–β interface. (B) A side view of two subunits, viewed from the channel lumen in the direction of the arrow in (A). Chain A (yellow) supplies the “−” side of the interface; chain B (green) supplies the “+” side of the interface. In β3 receptors, both sides of the interface are β subunits. In α1β3 receptors, the yellow chain is a β subunit, and the green chain is a β subunit or an α subunit. (C) The intersubunit interface at a higher resolution showing the side chains of amino acid residues probed in this study.