Abstract
Background
Allergen immunotherapy (AIT) is effective treatment for allergic diseases, and subcutaneous use of depigmented polymerized extracts may allow rapid up-dosing and safe therapy. To date, there is little information on their safety and clinical effects for children and adolescents with allergic disease.
Methods
We performed a retrospective survey of patient notes of 2927 children and adolescents across 136 centres who had received subcutaneous AIT (SCIT) with depigmented polymerized extracts to pollen or mite allergens for at least 1 yr to collect documentation on safety and clinical symptoms.
Results
16.3% percent of patients had local reactions, of these 148 were larger than 12 cm in diameter. Systemic reactions were documented in 1.6% of children and in 0.8% of adolescents. There were no documented cases of anaphylactic shock. There were significant reductions in the frequency of patients with recorded nasal symptoms over time of treatment. Moreover, the prescribing rate of rescue medication was reduced over the course of SCIT.
Conclusion
These ‘real-life’ data from a large retrospective analysis including 2927 children and adolescents with pollen- and/or mite-induced allergic rhinoconjunctivitis with/or without allergic asthma indicate that AIT with depigmented polymerized extracts is well tolerated, and they are compatible with clinical response.
Keywords: allergen, allergic rhinoconjunctivitis, allergen immunotherapy, non-interventional study, real-life data, depigmented polymerized
Prevalence of allergic rhinitis, conjunctivitis and/or allergic asthma has increased in the last decades 1,2. Allergen immunotherapy (AIT), in clinical use for more than 100 yr 3, is accepted on the basis of high-grade evidence as disease modifying treatment for these diseases 4–8. In Northern Europe, most of allergic patients are treated with extracts of grass or birch pollens, or house dust mites.
However, the use of subcutaneous AIT (SCIT) is limited to some extent by potential side effects which include anaphylaxis 9. An attempt to reduce this risk of SCIT was polymerization of natural (native) allergen extracts with chemicals such as glutaraldehyde, with the aim to reduce IgE binding yet to retain T-cell reactivity 10,11. This principle was later modified by treating the extracts with acid (‘depigmentation’) prior to polymerization 12,13. As shown in numerous DBPC trials, these depigmented polymerized extracts were well tolerated and efficacious in treating allergic adults and adolescents 14–18. However, there is little data for children. In addition, these controlled trials are performed in accordance with strict study protocols on carefully selected allergic patients and therefore should be supported with data from large-scale, post-marketing analysis of physician's routine clinical practice 19–21. Data from two prospective, post-marketing, multicentre surveys on safety and clinical effects of depigmented polymerized allergen extracts in adult and paediatric allergic patients have been previously published 19,20.
Here, our aim was to analyse the safety and clinical effects of depigmented polymerized allergen extracts in a large cohort of children and adolescents with pollen- and/or mite-induced allergic rhinoconjunctivitis with/or without allergic asthma.
Methods
Patients and clinical study design
This was a non-interventional, retrospective study including paediatric patients (children 5–11 yr old and adolescents 12–18 yr old) with allergic symptoms to pollen and mite allergens who received SCIT for at least 1 yr within the last 5 yr. The allergy was confirmed by medical history together with positive skin prick test or RAST class ≥2 (ImmunoCAP, ThermoFisher, Schwerte, Germany). Data were collected from patient's medical records by participating physicians in a total of 136 centres in Germany between October 2008 and April 2009, using a standardized data collection form. This included demographic characteristics, information on indications, types and amounts of SCIT given, and the course of the allergic condition in terms of symptoms and rescue treatment.
The study was approved by local ethics committee and notified to the relevant regulatory bodies. Only data were collected which had been recorded in the patient files during routine therapy.
Populations evaluated
Clinical symptoms over the course of treatment and safety were evaluated for the total group (all evaluable patients), children (patients 5–11 yr of age) and adolescents (patients 12–18 yr of age). Each age group was divided into subgroups (‘treatment groups’) according to the type of allergen. Treatment groups were Depigoid®-Bäume (‘Trees’; mixture of pollen from early-blooming trees, e.g. birch, alder, hazel), Depigoid®-Gräser (‘Grasses’; mixture of grass pollen, rye and cereal pollens, e.g. wheat, oat, barley, rye, timothy), Depigoid®-Bäume/Gräser (‘Trees/Grasses’; mixtures of pollen from the categories grasses and trees), Depigoid®-Kräuter (‘Weeds’; mixture of weeds, e.g. mugwort, plantain, wall pellitory, lambs quarter), Depigoid®-Milben (‘Mites’; mixture of Dermatophagoides pteronyssinus and D. farinae, D. pteronssinus, D. farinae, Euroglyphus maynei, Lepidoglyphus destructor, Tyrophagus putrescentiae, Acarus siro, D. microceras) and ‘multiple SCIT’ (application of different allergen classes other than ‘Trees/Grasses’, e.g. any pollen/mite, grass pollen/weed pollen SCITs). All extracts were manufactured by Laboratorios Leti, SL, Tres Cantos, Spain. Patients’ demographics sensitization and extracts used are given in Table1.
Table 1.
Demographic and clinical details of children and adolescents
| Children (aged 5–11) Total 1678 | Adolescents (aged 12–18) 1237 | |
|---|---|---|
| Gender | 1018 male | 728 male |
| Sensitization | ||
| Grass pollen | 1070 | 837 |
| Tree pollen | 783 | 608 |
| Mites | 698 | 562 |
| Weeds | 172 | 170 |
| Disease | ||
| Rhinoconjunctivitis | 1385 | 1055 |
| Asthma | 958 | 586 |
| Other | 202 | 112 |
| Allergen extract used for SCIT | ||
| Grass pollen | 488 | 374 |
| Tree pollen | 489 | 340 |
| Tree/grass mix | 174 | 127 |
| Mites | 470 | 332 |
| Multi-allergen | 56 | 61 |
Data are absolute numbers for each variable as recorded in case records. Allergic disease is that recorded, some were multiple. Multiple SCIT refers to patients treated with mixed extracts for three pollens, or mites plus pollens. Four additional patients have received SCIT with weed-extracts, but have not been displayed and considered for further discussion due to the limited group size.
Evaluation of safety
The safety of the treatment was assessed by the number and frequency of local and systemic adverse drug reactions (ADRs), serious adverse drug reactions (sADRs) and the related measures. A standardized assessment was used for reactions considered related to immunotherapy (Table2). If adrenaline was given, detailed description of the adverse event was required.
Table 2.
Adverse reactions to immunotherapy
| Recording |
|---|
| Local reactions |
| Grade 1 (L1): local swelling or nodules <12 cm in diameter |
| Grade 2 (L2): local reaction ≥12 cm in diameter |
| Systemic reactions |
| Grade 1 (S1): exacerbation of patient-specific symptoms (mild allergy: itchy eyes, sneezing, cough, atopic eczema) |
| Grade 2 (S2): moderate allergic reaction (wheezing, breathlessness, angioedema, generalized urticaria) |
| Grade 3 (S3): anaphylactic shock |
| Allergen | Children | Adolescents | ||||||
|---|---|---|---|---|---|---|---|---|
| L1 | L2 | S1 | S2 | L1 | L2 | S1 | S2 | |
| Grass pollen | (488) 228 | 28 | 22 | 4 | (374) 104 | 19 | 5 | 0 |
| Tree pollen | (489) 196 | 17 | 12 | 1 | (340) 75 | 18 | 2 | 0 |
| Tree/grass | (174) 89 | 11 | 0 | 0 | (127) 26 | 5 | 0 | 0 |
| Mites | (470) 153 | 12 | 7 | 1 | (332) 114 | 20 | 0 | 8 |
| Mixed | (56) 48 | 11 | 4 | 1 | (61) 31 | 7 | 0 | 0 |
Numbers of recorded local or systemic reactions for each allergen extract (total number of patients treated with each extract shown in brackets). There were no recorded cases of anaphylactic shock.
Dose reduction occurred 153 times in children and 67 times in adolescents following LR<10 cm, 31 times in children and 10 times in adolescents following large local reactions, eight times in children following mild systemic symptoms and two times in children following moderate systemic symptoms.
Evaluation of clinical symptoms over the course of treatment
The presence of eye, nose, lung and skin symptoms was recorded at different time points, and the prescription of symptomatic medication with time was used to assess clinical effects of SCIT.
Statistics
Data were analysed by descriptive statistics using SAS software (SAS Institute Inc., Cary, NC, USA; version 9.2). Continuous numeric values or values scaled on intervals were expressed as number of evaluable values, mean, standard deviation, median, minimum and maximum. Ordinal or categorical values were expressed in absolute and relative frequencies. Patients for which the respective parameters were missing were excluded from the evaluation of relative frequencies. Frequencies of patients reporting symptoms for each year of treatment were compared by chi-square test, comparing year one with baseline, year two with year one and year three with year two.
Results
Demographic data are shown in Table1.
In the up-dosing phase of the SCIT, a mean of 4.7 injections was given in the total group which is in accordance with the recommended up-dosing regimen for these products. Patients receiving multiple SCIT required 1.5–2 times the number of injections to reach the maintenance dose.
The mean treatment duration for the study populations of the SCIT was 2.9 yr (SD 0.89 yr).
Safety
Numbers of recorded treatment-related local and systemic ADRs for each allergen extract are shown in Table2.
In ten times adolescents received subcutaneous adrenaline for large local reactions without systemic reactions. Six of these were during up-dosing (four receiving mite extract, one grass pollen and one tree pollen), three were in year two of treatment (two receiving mite extract and one tree pollen), and one was in year three of treatment with grass pollen extract. One of these patients elected to discontinue treatment, and the rest continued after dose reduction for the next visit. Most systemic reactions occurred during up-dosing or the first year of treatment. None of the systemic reactions were treated with adrenaline. In seven times in children and in two times in adolescents intravenous antihistamines were given for systemic reactions, and in six times in children and in two times in adolescents intravenous steroids were given for systemic reactions. All of these patients continued treatment after dose reduction. The frequency of patients affected by ADR was twice as high in the ‘multiple SCIT’ group as in the other treatment groups. Most ADRs were observed at the beginning of the SCIT and reached a minimum by the end of the treatment. Seven per cent of patients discontinued treatment (5.3% of children and 9.3% of adolescents). In no case was SCIT discontinued by the doctor because of serious ADR. Most discontinuations were due to the patient's/parent's wishes or other reasons, which included change of the doctor, relocation, concomitant symptoms/diseases, poor success of the treatment and non-compliance. In seven children and four adolescents the treatment was discontinued due to ADRs.
Clinical assessment
Before treatment, most patients’ allergic symptoms were nasal (90.0%), followed by eyes (76.3%) and lungs (61.0%). In fewer patients (14.1%), the skin was affected by allergic symptoms. More children than adolescents suffered from lung symptoms (65.9% vs. 54.1%). Data for changes in the recorded frequency of nasal symptoms over the course of treatment are presented in Table3. Numbers of patients reporting nasal symptoms were significantly reduced over the course of treatment (using chi-square test; Table3). Data on eye, lung and skin symtoms are not shown.
Table 3.
Numbers of patients reporting nasal symptoms over the course of immunotherapy
| Treatment | Nasal symptoms | |||||
|---|---|---|---|---|---|---|
| Children | Adolescents | |||||
| Year | Symptoms | Year | Symptoms | |||
| Yes | No | Yes | No | |||
| Grass pollen | 0 | 458 | 30 | 0 | 348 | 25 |
| 1 | 436 | 41 | 1 | 318 | 50*** | |
| 2 | 313 | 69**** | 2 | 210 | 75**** | |
| 3 | 172 | 72**** | 3 | 115 | 73*** | |
| Tree pollen | 0 | 470 | 19 | 0 | 321 | 19 |
| 1 | 428 | 52**** | 1 | 295 | 41*** | |
| 2 | 306 | 65** | 2 | 203 | 69**** | |
| 3 | 169 | 53 | 3 | 104 | 68*** | |
| Tree/Grass | 0 | 158 | 16 | 0 | 119 | 8 |
| 1 | 145 | 24 | 1 | 111 | 9 | |
| 2 | 101 | 39*** | 2 | 82 | 20** | |
| 3 | 67 | 29 | 3 | 40 | 30*** | |
| Mites | 0 | 361 | 107 | 0 | 273 | 59 |
| 1 | 304 | 154**** | 1 | 242 | 81* | |
| 2 | 230 | 197**** | 2 | 205 | 107* | |
| 3 | 137 | 164* | 3 | 126 | 101* | |
| Mixed | 0 | 53 | 3 | 0 | 58 | 3 |
| 1 | 49 | 6 | 1 | 53 | 8 | |
| 2 | 40 | 9 | 2 | 46 | 12 | |
| 3 | 26 | 11 | 3 | 35 | 9 | |
Statistical comparison by chi-square test of distribution for each year with preceding year (*p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001).
The prescription of anti-allergic co-medication decreased for all allergen groups (see Fig.1). This was seen for oral and topical antihistamines, topical (nasal and inhaled) corticosteroids and for topical cromoglycate.
Figure 1.
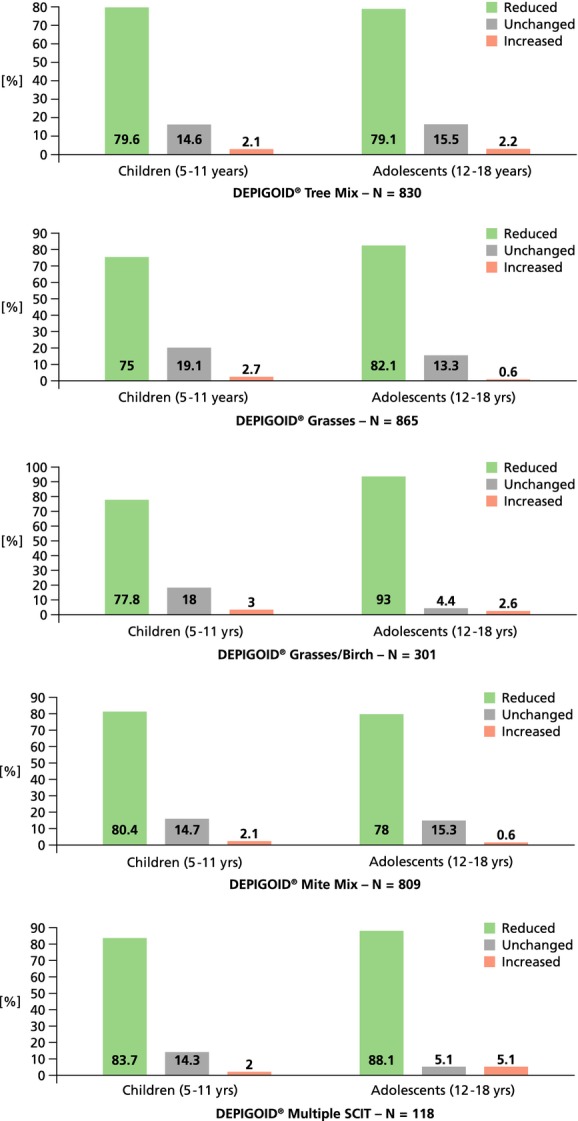
Percentage of patients with reduction of anti-allergic co-medication (and completion of a treatment course of at least 1 year of AIT).
Discussion
In this study, we present a retrospective survey of safety and clinical effects of subcutaneous AIT with depigmented polymerized preparations containing pollen and/or mite allergens in a large cohort of nearly 3000 children and adolescents. The data suggest this treatment was well tolerated and show a significant reduction in the proportion of patients with symptoms over the years of treatment.
In a previous analysis of data collected prospectively 766 patients (including 17% children and 24% adolescents), the safety profile of AIT using depigmented polymerized extracts of pollen or mite allergens was excellent with 54 local reactions and 16 systemic reactions (15 grade 2 and 1 grade 3) 19. In a subsequent prospective survey of 768 patients (210 children and adolescents) with allergic rhinoconjunctivitis and asthma receiving AIT with these preparations under daily practice conditions, our group found both a good safety profile and a reduction of symptoms and need of concomitant anti-allergic medication 20. In that survey, there were 14 local reactions and 27 systemic reactions (20 grade 1, seven grade 2 and no grade 3 or 4 reactions). In a study comparing rush (two injections to reach maintenance dose at visit one) vs. conventional up-dosing of AIT with depigmented polymerized extracts, the rates of systemic reactions were 5.8% for rush AIT and 2% for conventional up-dosing (all grade 2 or less) with rates of local reactions of 24% and 11%, respectively 14. However, to date, clinical data on AIT with depigmented polymerized extracts in children and adolescents are still limited.
In our study, 79 large local reactions (≥ 12 cm in diameter) and 714 smaller reactions (< 12 cm) were recorded in a total number of 1677 children treated with similar figures for 1234 adolescents treated (69 large local reactions, 350 smaller local reactions) which is higher than the two prospective series referenced above. However, these numbers are in line with rates of local reactions seen in double-blind placebo-controlled studies of depigmented polymerized extracts 15–18. In the present study, 7.4% of all local reactions were treated with oral antihistamines and/or topical corticosteroids and 23.2% of all local reactions lead to a dose reduction in children and 18.4% in adolescents, but treatment was continued. Surprisingly, subcutaneous adrenaline was given 10 times in adolescents with large local reactions, but no recorded systemic reaction. Most of these were during up-dosing and most were being treated for mite allergy. Systemic reactions were recorded in 27 children (1.6%) and 10 adolescents (0.8%) and were mostly mild. There was a higher rate of local and systemic reactions in children compared with adolescents. The higher frequency of patients affected with ADR in the ‘multiple SCIT’ group may be explained by the higher numbers of injections, due to the parallel administration of two preparations, one on each arm, and not by a lower tolerability of the therapy. Further data are required on whether local reactions are more troublesome in children treated with AIT, but overall, the safety profile here is reassuring.
Data in this report were collected retrospectively from patient notes rather than from a placebo-controlled study. One cannot therefore firmly ascribe any documented reduction in the presence of symptoms to the medication given, and the descriptive data are less rigorous than a standardized daily symptom and medication diary as used in prospective placebo-controlled trials 22. Nonetheless, the significant reductions seen here in proportions of children and adolescents with recorded nasal symptoms over the course of treatment are compatible with an improvement due to immunotherapy and are in accordance with data from randomized, double-blind placebo-controlled trials of depigmented polymerized extracts for AIT performed in adolescents and adults 15–18. Overall, there was a reduction in those reporting symptoms, and a reduction in use of rescue medication, which was seen for each of the allergen treatment groups in both children and adolescents. Strengths of the data presented here are that they present real-life clinical practice, and a very large group of patients.
When the trend for reduced proportion with recorded symptoms was analysed for treatment year, the data suggested that there might be a year-on-year improvement with continuing SCIT with depigmented polymerized extracts. Such a finding would be in accord with data from other studies of both SCIT and SLIT 4–6. Further research is required to determine the optimal dose and duration of SCIT in paediatric patients. Of note, some caution should be applied to this interpretation as the same patients were not studied over a 3-yr time course. Controlled, prospective long-term studies are warranted.
The group treated with multiple allergen SCIT appeared to respond equally well to treatment as those treated with a single allergen, although it is of note that more adverse events were reported in this group. This finding is at variance with previous reports and reviews, suggesting that mixed multi-allergen SCIT might be less effective than single allergen dosing as dose reductions are required for each allergen in the mixture 18,23. Here, most multi-allergen regimens were of two allergens given one in each arm without dose reduction rather than mixtures of multiple allergens used in other studies, and this might explain the apparent difference. The data here need to be confirmed by prospective controlled trials. A recent pilot study suggests it may be safe to mix depigmented polymerized extracts without dose reduction (data on file, Laboratorios Leti, Madrid, Spain).
For our analysis, we selected children and adolescents who had completed at least 1 yr of AIT. It is possible that such selection may have introduced bias as any children not responding to AIT might discontinue earlier than 1 yr. Prescribing data (data on file, Leti Pharma GmbH, Witten, Germany) emphasize that the dropout rate during the first year of AIT with depigmented polymerized extracts during the study period was around 10%. We therefore suggest that this bias was unlikely to have an important effect on the study conclusion, although prospective controlled data are required. Similarly, it is possible that the study design did not detect delayed adverse events treated at other medical centres, although, as adverse events were always asked about before giving the next dose, these should have been detected and recorded.
In summary, this large retrospective survey analysing ‘real-life’ data of almost 3000 children and adolescents with rhinoconjunctivitis with/or without asthma suggests that AIT with depigmented polymerized pollen or mite extracts is well tolerated as well as reducing symptoms.
Acknowledgments
Leti Pharma GmbH, Witten, Germany, sponsored this analysis, assumed overall responsibility and was involved in analysis design and conduct, data management, collection, analysis, interpretation, and publication of the study. The authors thank patients and investigators in this study. The investigators received remuneration from LETI for their time spent with each patient, all trial procedures and staff fees were in conjunction with the ICH Guideline for Good Clinical Practice. The authors thank MedPharmTec Services and Carmen Theek, Herdecke, Germany, for data analysis. We moreover thank Franziska T. Allinghausen (ACCOVION gmbH) for assistance in medical writing.
Financial disclosure
O. Pfaar (OP) has received research grants for his institution from ALK-Abelló (Germany/Denmark), Allergopharma (Germany), Stallergenes (Germany/France), HAL Allergy (Germany/the Netherlands), Artu Biologicals (the Netherlands), Allergy Therapeutics/Bencard (UK/Germany), Hartington (Spain), Lofarma (Italy), Novartis/Leti (Germany/Spain), GlaxoSmithKline (UK/Germany), Essex Pharma (Germany), Cytos (Switzerland), Curalogic (Denmark), Roxall (Germany), Biomay (Austria), Thermo Fisher (Germany), Circassia (UK), European Union (FP-7 Health-2013 Innovation 1), Biotech Tools s.a. (Belgium), and Meda Pharma GmbH (Germany); and/or he has served as an advisor and on speakers' bureaus for some of the aforementioned companies. OP has received travel grants from HAL Allergy (the Netherlands/Germany) and Allergopharma (Germany), and he is a consultant for Bencard (Germany), HAL Allergy (the Netherlands), Novartis/Leti (Germany), Meda (Germany), ALK-Abelló (Germany/Denmark), Allergopharma (Germany), Biotech Tools s.a. (Belgium), GfK Bridgehead (UK), Navigant Consulting (USA), Sanofi (USA), Guidepoint Global Advisors (USA), Thermo Fisher (Germany) and Stallergenes (Germany/France); he is Scientific Board Member of Mobile Chamber Experts (MCX), a GA2LEN Partner. OP is the current chairman of the Immunotherapy Interest Group (IT IG) of the European Academy of Allergy and Clinical Immunology (EAACI) and is the secretary of the ENT section of Deutsche Gesellschaft für Allergologie und Klinische Immunologie (DGAKI). He has received grants for the “Spezifische Immuntherapie”-award 2014 and the “Nachwuchsförderpreis”- award 2010 of the DGAKI. He is co-editor and an author of the textbook “Allergien bei Kindern und Jugendlichen” (publisher: Schattauer-Verlag, Germany) and author of different chapters of “Allergologie- Handbuch” (publisher: Schattauer-Verlag, Germany) and has received payment for development of educational presentations from GlaxoSmithKline (Germany), Bencard (Germany), and Novartis (Germany). Angelika Sager (AS) is an employee of Leti Pharma GmbH, Germany' Douglas S. Robinson (DSR) is a consultant for Laboratorios Leti, Spain.
Authors contribution
OP and DSR have substantially contributed to the manuscript from the first draft stage. AS was the sponsor's project manager of the trial and sponsor's medical director and was involved in the study design and conduct of the analysis. She furthermore contributed to the manuscript by reviewing and commenting as well as critically revising where applicable.
References
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- Noon L, Cantar B. Prophylactic inoculation against hay fever. Lancet. 1911;1:1572–3. [Google Scholar]
- Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936. doi: 10.1002/14651858.CD001936.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–6. doi: 10.1016/j.jaci.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Burks AW, Calderon MA, Casale T, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–96. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- Calderon MA, Gerth van Wijk R, Eichler I, et al. Perspectives on allergen-specific immunotherapy in childhood: an EAACI position statement. Pediatr Allergy Immunol. 2012;23:300–6. doi: 10.1111/j.1399-3038.2012.01313.x. [DOI] [PubMed] [Google Scholar]
- Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen-specific immunotherapy in lgE mediated allergic diseases - S2k Guideline of the German Society for Allergology and Clinical lmmunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and lmmunology (ÖGAl), the Swiss Society for Allergy and lmmunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD) Allergo J lnt. 2014;23:282–319. doi: 10.1007/s40629-014-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukantz SC, Bagg AS, Lockey RF. Adverse effects and fatalities associated with subcutaneous allergen immunotherapy. Clin Allergy Immunol. 2008;21:455–68. [PubMed] [Google Scholar]
- Marsh DG. Preparation and properties of, allergoids, derived from native pollen allergens by mild formalin treatment. Int Arch Allergy Appl Immunol. 1971;41:199–215. doi: 10.1159/000230518. [DOI] [PubMed] [Google Scholar]
- Grammer LC, Shaughnessy MA, Patterson R. Modified forms of allergen immunotherapy. J Allergy Clin Immunol. 1985;76:397–401. doi: 10.1016/0091-6749(85)90661-x. [DOI] [PubMed] [Google Scholar]
- Casanovas M, Gomez MJ, Carnes J, Fernandez-Caldas E. Skin tests with native, depigmented and glutaraldehyde polymerized allergen extracts. J Investig Allergol Clin Immunol. 2005;15:30–6. [PubMed] [Google Scholar]
- Fernandez-Caldas E, Gallego M, Carnes J, Iraola V. Enzymatic activity of Dermatophagoides pteronyssinus extracts after acidic treatment. Int Arch Allergy Immunol. 2008;145:298–304. doi: 10.1159/000110888. [DOI] [PubMed] [Google Scholar]
- Brehler R, Klimek L, Pfaar O, Hauswald B, Worm M, Bieber T. Safety of a rush immunotherapy build-up schedule with depigmented polymerized allergen extracts. Allergy Asthma Proc. 2010;31:e31–8. doi: 10.2500/aap.2010.31.3334. [DOI] [PubMed] [Google Scholar]
- Hoiby AS, Strand V, Robinson DS, Sager A, Rak S. Efficacy, safety, and immunological effects of a 2-year immunotherapy with Depigoid birch pollen extract: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy. 2010;40:1062–70. doi: 10.1111/j.1365-2222.2010.03521.x. [DOI] [PubMed] [Google Scholar]
- Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented-polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65:1614–21. doi: 10.1111/j.1398-9995.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- Pfaar O, Urry Z, Robinson DS, et al. A randomized placebo-controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy*. Allergy. 2012;67:272–9. doi: 10.1111/j.1398-9995.2011.02736.x. [DOI] [PubMed] [Google Scholar]
- Pfaar O, Biedermann T, Klimek L, Sager A, Robinson DS. Depigmented-polymerized mixed grass/birch pollen extract immunotherapy is effective in polysensitized patients. Allergy. 2013;68:1306–13. doi: 10.1111/all.12219. [DOI] [PubMed] [Google Scholar]
- Casanovas M, Martin M, Jimenez C, Cabellero R, Fernandez-Caldas E. Safety of immunotherapy with therapeutic vaccines containing depigmented and polymerized allergen extracts. Clin Exp Allergy. 2007;37:434–40. doi: 10.1111/j.1365-2222.2007.02667.x. [DOI] [PubMed] [Google Scholar]
- Pfaar O, Klimek L, Sager A, Brautigam M. Safety of a depigmented, polymerized vaccine for the treatment of allergic rhinoconjunctivitis and allergic asthma. Am J Rhinol Allergy. 2010;24:220–5. doi: 10.2500/ajra.2010.24.3437. [DOI] [PubMed] [Google Scholar]
- Crivellaro M, Senna GE, Pappacoda A, et al. Safety of ultrashort-term sit with pollen allergoids adjuvanted by monophosphoryl lipid A: a prospective Italian survey. Eur Ann Allergy Clin Immunol. 2011;43:58–60. [PubMed] [Google Scholar]
- Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–67. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- Calderon MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple-allergen and single-allergen immunotherapy strategies in polysensitized patients: looking at the published evidence. J Allergy Clin Immunol. 2012;129:929–34. doi: 10.1016/j.jaci.2011.11.019. [DOI] [PubMed] [Google Scholar]


